Misoprostol
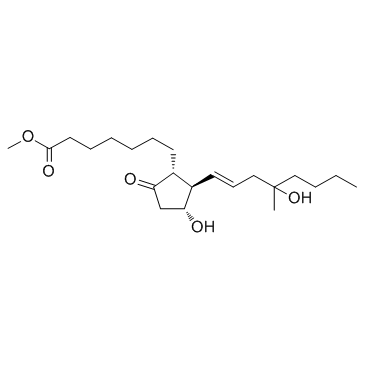
Misoprostol structure
|
Common Name | Misoprostol | ||
|---|---|---|---|---|
| CAS Number | 59122-46-2 | Molecular Weight | 382.534 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 497.3±45.0 °C at 760 mmHg | |
| Molecular Formula | C22H38O5 | Melting Point | 261-263°C | |
| MSDS | USA | Flash Point | 160.4±22.2 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of MisoprostolMisoprostol(SC29333) is a synthetic prostaglandin E1 (PGE1) analog that is used to prevent gastric ulcers, to treat missed miscarriage, to induce labor, and to induce abortion.Target: Prostaglandin ReceptorMisoprostol is a synthetic analog of natural prostaglandin E1. It produces a dose-related inhibition of gastric acid and pepsin secretion, and enhances mucosal resistance to injury. It is an effective anti-ulcer agent and also has oxytocic properties. Misoprostol seems to inhibit gastric acid secretion by a direct action on the parietal cells through binding to the prostaglandin receptor. Administration of misoprostol to EP3+/+ and EP3-/- mice showed similar levels of infarct rescue, indicating that misoprostol protection was not mediated through the EP3 receptor. Taken together, these findings suggest a novel function for misoprostol as a protective agent in cerebral ischemia acting via the PGE(2) EP2 and/or EP4 receptors [1, 2]. |
| Name | Misoprostol |
|---|---|
| Synonym | More Synonyms |
| Description | Misoprostol(SC29333) is a synthetic prostaglandin E1 (PGE1) analog that is used to prevent gastric ulcers, to treat missed miscarriage, to induce labor, and to induce abortion.Target: Prostaglandin ReceptorMisoprostol is a synthetic analog of natural prostaglandin E1. It produces a dose-related inhibition of gastric acid and pepsin secretion, and enhances mucosal resistance to injury. It is an effective anti-ulcer agent and also has oxytocic properties. Misoprostol seems to inhibit gastric acid secretion by a direct action on the parietal cells through binding to the prostaglandin receptor. Administration of misoprostol to EP3+/+ and EP3-/- mice showed similar levels of infarct rescue, indicating that misoprostol protection was not mediated through the EP3 receptor. Taken together, these findings suggest a novel function for misoprostol as a protective agent in cerebral ischemia acting via the PGE(2) EP2 and/or EP4 receptors [1, 2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 497.3±45.0 °C at 760 mmHg |
| Melting Point | 261-263°C |
| Molecular Formula | C22H38O5 |
| Molecular Weight | 382.534 |
| Flash Point | 160.4±22.2 °C |
| Exact Mass | 382.271912 |
| PSA | 83.83000 |
| LogP | 2.91 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.525 |
| InChIKey | OJLOPKGSLYJEMD-URPKTTJQSA-N |
| SMILES | CCCCC(C)(O)CC=CC1C(O)CC(=O)C1CCCCCCC(=O)OC |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H360 |
| Precautionary Statements | P201-P301 + P310 + P330-P308 + P313 |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R60;R61;R25 |
| Safety Phrases | S53-S22-S36/37/39-S45 |
| RIDADR | UN 2810 6.1/PG 3 |
| WGK Germany | 2 |
| RTECS | UK8390000 |
| Hazard Class | 6.1 |
| Precursor 10 | |
|---|---|
| DownStream 0 | |
|
Bleeding diathesis and gastro-duodenal ulcers in inherited cytosolic phospholipase-A2 alpha deficiency.
Thromb. Haemost. 112(6) , 1182-9, (2014) Arachidonic acid (AA), when cleaved from phospholipids by cytosolic phospholipase A2 alpha (cPLA2a), generates eicosanoids, with pro-hemostatic, pro-inflammatory, vasoactive and gastro-protective func... |
|
|
Mucosal protective agents prevent exacerbation of NSAID-induced small intestinal lesions caused by antisecretory drugs in rats.
J. Pharmacol. Exp. Ther. 348(2) , 227-35, (2014) Antisecretory drugs such as histamine H₂-receptor antagonists and proton pump inhibitors are commonly used for the treatment of upper gastrointestinal mucosal lesions induced by nonsteroidal anti-infl... |
|
|
Inhibition of TNF-α, and NF-κB and JNK pathways accounts for the prophylactic action of the natural phenolic, allylpyrocatechol against indomethacin gastropathy
Biochim. Biophys. Acta 1830(6) , 3776-86, (2013) Background The gastro-intestinal disorders, induced by the NSAIDs including indomethacin (IND) remain unresolved medical problems. Herein, we disclose allylpyrocatechol (APC) as a potential agent agai... |
| Methyl (11α,13E)-11,16-dihydroxy-16-methyl-9-oxoprost-13-en-1-oate |
| MISOPROSTOL ACID |
| SC 2933 |
| CYTOTEC |
| Misoprosto |
| MFCD07772001 |
| Prost-13-en-1-oic acid, 11,16-dihydroxy-16-methyl-9-oxo-, methyl ester, (11α,13E)- |
| Misoprostil |
| UNII:0E43V0BB57 |
| (11a,13E)-(±)-11,16-Dihydroxy-16-methyl-9-oxoprost-13-en-1-oic Acid Methyl Ester |
| Misoprostol |
| methyl 7-{(1R,2R,3R)-3-hydroxy-2-[(1E)-4-hydroxy-4-methyloct-1-en-1-yl]-5-oxocyclopentyl}heptanoate |
| Methyl-7-{(1R,2R,3R)-3-hydroxy-2-[(1E)-4-hydroxy-4-methyloct-1-en-1-yl]-5-oxocyclopentyl}heptanoat |
| Misogon |
| DL-MISOPROSTOL |
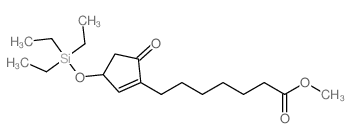 CAS#:112713-92-5
CAS#:112713-92-5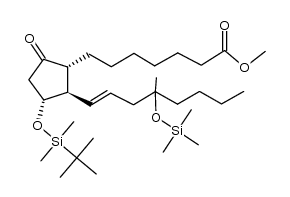 CAS#:104334-05-6
CAS#:104334-05-6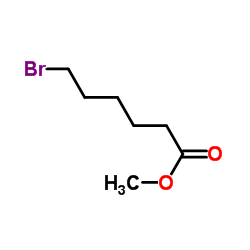 CAS#:14273-90-6
CAS#:14273-90-6 CAS#:88462-11-7
CAS#:88462-11-7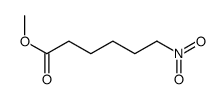 CAS#:13154-40-0
CAS#:13154-40-0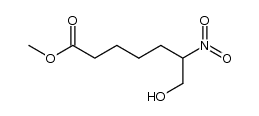 CAS#:112329-43-8
CAS#:112329-43-8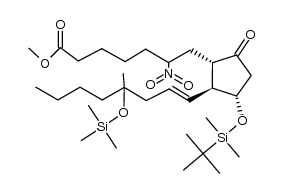 CAS#:101642-17-5
CAS#:101642-17-5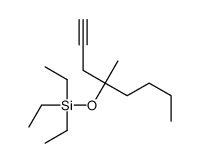 CAS#:58682-77-2
CAS#:58682-77-2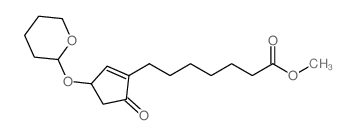 CAS#:40098-24-6
CAS#:40098-24-6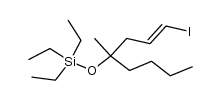 CAS#:58682-78-3
CAS#:58682-78-3
