Methyl 3-indolecarboxylate
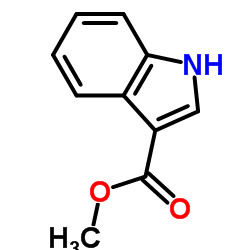
Methyl 3-indolecarboxylate structure
|
Common Name | Methyl 3-indolecarboxylate | ||
|---|---|---|---|---|
| CAS Number | 942-24-5 | Molecular Weight | 175.184 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 331.7±15.0 °C at 760 mmHg | |
| Molecular Formula | C10H9NO2 | Melting Point | 149-152 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 154.4±20.4 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Methyl 3-indolecarboxylateMethyl indole-3-carboxylate is a natural product isolated from Sorangium cellulosum strain Soce895. Methyl indole-3-carboxylate shows a weak activity against the Gram-positive Nocardia sp with a MIC value of 33.33 μg/mL[1]. |
| Name | Methyl indole-3-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Methyl indole-3-carboxylate is a natural product isolated from Sorangium cellulosum strain Soce895. Methyl indole-3-carboxylate shows a weak activity against the Gram-positive Nocardia sp with a MIC value of 33.33 μg/mL[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 331.7±15.0 °C at 760 mmHg |
| Melting Point | 149-152 °C(lit.) |
| Molecular Formula | C10H9NO2 |
| Molecular Weight | 175.184 |
| Flash Point | 154.4±20.4 °C |
| Exact Mass | 175.063324 |
| PSA | 42.09000 |
| LogP | 2.54 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.639 |
| InChIKey | QXAUTQFAWKKNLM-UHFFFAOYSA-N |
| SMILES | COC(=O)c1c[nH]c2ccccc12 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Regioselective dibromination of methyl indole-3-carboxylate and application in the synthesis of 5,6-dibromoindoles.
Org. Biomol. Chem. 9(14) , 5021-3, (2011) Treatment of methyl indole-3-carboxylate with bromine in acetic acid gives methyl 5,6-dibromoindole-3-carboxylate regioselectively, from which the parent 5,6-dibromoindole can be accessed via a one-po... |
|
|
Nannozinones and sorazinones, unprecedented pyrazinones from myxobacteria.
J. Nat. Prod. 77(11) , 2545-52, (2014) Nannozinones A (1) and B (2) were discovered as metabolites of the recently isolated Nannocystis pusilla strain MNa10913 belonging to the poorly studied myxobacterial family Nannocystaceae. In contras... |
|
|
Methyl indole-3-carboxylate. Hu SC, et al.
Acta Crystallogr. Sect. E Struct. Rep. Online 61(6) , o1654-o1656, (2005)
|
|
Name: Inverse agonist activity at Gal4-fused human Nurr1 LBD expressed in HEK293T cells co-...
Source: ChEMBL
Target: Nuclear receptor subfamily 4 group A member 2
External Id: CHEMBL4840094
|
|
Name: Inverse agonist activity at Nurr1 (unknown origin) assessed as remaining activity rel...
Source: ChEMBL
Target: Nuclear receptor subfamily 4 group A member 2
External Id: CHEMBL5141087
|
|
Name: Inverse agonist activity at Gal4-fused human Nurr1 LBD expressed in HEK293T cells co-...
Source: ChEMBL
Target: Nuclear receptor subfamily 4 group A member 2
External Id: CHEMBL4840093
|
|
Name: Chemical Probes of Kaposi's Sarcoma Herpes Virus Latent Infection
Source: ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
Target: ORF 73 [Human herpesvirus 8 type M]
External Id: HMS791
|
|
Name: A screen for compounds that inhibit the activity of LtaS in Staphylococcus aureus
Source: ICCB-Longwood/NSRB Screening Facility, Harvard Medical School
External Id: HMS979
|
|
Name: Inhibition of mushroom tyrosinase using tyrosine as substrate pretreated for 5 mins f...
Source: ChEMBL
Target: Polyphenol oxidase 2
External Id: CHEMBL4186864
|
|
Name: Antimicrobial activity against Candida albicans 1665 after 24 to 48 hrs by serial dil...
Source: ChEMBL
Target: Candida albicans
External Id: CHEMBL3383960
|
|
Name: Antimicrobial activity against Saccharomyces cerevisiae 70449 after 24 to 48 hrs by s...
Source: ChEMBL
Target: Saccharomyces cerevisiae
External Id: CHEMBL3383959
|
|
Name: Antimicrobial activity against Trichosporon oleaginosus 11815 after 24 to 48 hrs by s...
Source: ChEMBL
Target: NON-PROTEIN TARGET
External Id: CHEMBL3376582
|
|
Name: Antimicrobial activity against Wickerhamomyces anomalus 6766 after 24 to 48 hrs by se...
Source: ChEMBL
Target: Wickerhamomyces anomalus
External Id: CHEMBL3383961
|
| Methyl 1H-indole-3-carboxylate |
| Indole-3-carboxylic acid, methyl ester |
| 1H-Indole-3-carboxylic acid, methyl ester |
| METHYL INDOLE-3-CARBOXYLATE |
| MFCD00189407 |
 CAS#:141-78-6
CAS#:141-78-6 CAS#:338760-26-2
CAS#:338760-26-2 CAS#:131424-24-3
CAS#:131424-24-3 CAS#:128942-87-0
CAS#:128942-87-0 CAS#:120-72-9
CAS#:120-72-9 CAS#:67-56-1
CAS#:67-56-1 CAS#:76-02-8
CAS#:76-02-8 CAS#:108438-44-4
CAS#:108438-44-4 CAS#:186581-53-3
CAS#:186581-53-3 CAS#:106202-36-2
CAS#:106202-36-2 CAS#:109175-08-8
CAS#:109175-08-8 CAS#:109175-09-9
CAS#:109175-09-9 CAS#:32387-21-6
CAS#:32387-21-6 CAS#:141102-07-0
CAS#:141102-07-0![methyl 6,7,8,9-tetrahydropyrido[1,2-a]indole-10-carboxylate structure](https://image.chemsrc.com/caspic/108/22766-24-1.png) CAS#:22766-24-1
CAS#:22766-24-1 CAS#:244090-32-2
CAS#:244090-32-2 CAS#:876-72-2
CAS#:876-72-2 CAS#:98600-34-1
CAS#:98600-34-1 CAS#:81038-38-2
CAS#:81038-38-2