Trichloroacetyl chloride

Trichloroacetyl chloride structure
|
Common Name | Trichloroacetyl chloride | ||
|---|---|---|---|---|
| CAS Number | 76-02-8 | Molecular Weight | 181.833 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 118.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C2Cl4O | Melting Point | -57 °C | |
| MSDS | Chinese USA | Flash Point | 31.0±26.5 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
| Name | Trichloroacetyl Chloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 118.0±0.0 °C at 760 mmHg |
| Melting Point | -57 °C |
| Molecular Formula | C2Cl4O |
| Molecular Weight | 181.833 |
| Flash Point | 31.0±26.5 °C |
| Exact Mass | 179.870331 |
| PSA | 17.07000 |
| LogP | 2.39 |
| Vapour Pressure | 17.0±0.2 mmHg at 25°C |
| Index of Refraction | 1.501 |
| InChIKey | PVFOMCVHYWHZJE-UHFFFAOYSA-N |
| SMILES | O=C(Cl)C(Cl)(Cl)Cl |
| Stability | Stable. Reacts violently with water. Incompatible with alcohols, oxidizing agents, strong bases. |
| Water Solubility | reacts violently |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H314-H330 |
| Supplemental HS | Reacts violently with water. |
| Precautionary Statements | P260-P280-P284-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | T+:Verytoxic; |
| Risk Phrases | R14;R22;R26;R29;R35 |
| Safety Phrases | S26-S28-S36/37/39-S45-S8-S28A-S23 |
| RIDADR | UN 2442 8/PG 2 |
| WGK Germany | 3 |
| RTECS | AO7140000 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 2914700090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Enantioselective synthesis of dihydro-1H-benzindoles.
J. Org. Chem. 78(7) , 3379-83, (2013) The first examples of dihydro-1H-benzindoles by enantioselective γ-lactamization reaction of naphthyl sulfilimines with trichloroacetyl chloride in the presence of ZnCu as catalyst (≥98:2 er and 65-80... |
|
|
Synthesis of 3-alkylbenzoxazolones from N-alkyl-N-arylhydroxylamines by contiguous O-trichloroacetylation, trichloroacetoxy ortho-shift, and cyclization sequence.
J. Org. Chem. 78(23) , 11935-47, (2013) Benzoxazolone pharmacophore is present in clinical pharmaceuticals, drug candidates, and many compounds having a wide spectrum of biological activities. The methods available for the synthesis of benz... |
|
|
Interindividual variability in P450-dependent generation of neoantigens in halothane hepatitis.
Chem. Biol. Interact. 116(1-2) , 123-41, (1998) Halothane hepatitis occurs because susceptible patients mount immune responses to trifluoroacetylated protein antigens, formed following cytochrome P450-mediated bioactivation of halothane to trifluor... |
| Trichloroacetochloride |
| Acetyl chloride, 2,2,2-trichloro- |
| Acetyl chloride, trichloro- |
| CCl3COCl |
| EINECS 200-926-7 |
| TRICHLOROACETIC ACID CHLORIDE |
| Trichloroacetyl chloride [UN2442] [Corrosive] |
| MFCD00000792 |
| Trichloroacetyl chloride |
| 2,2,2-Trichloroethanoyl chloride |
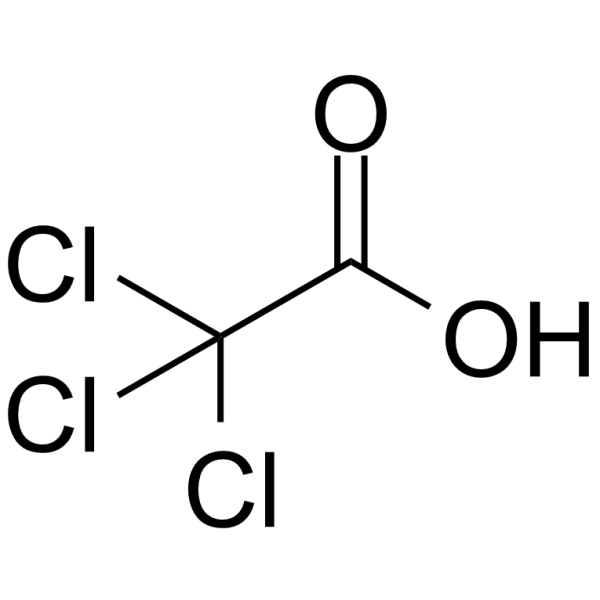 CAS#:76-03-9
CAS#:76-03-9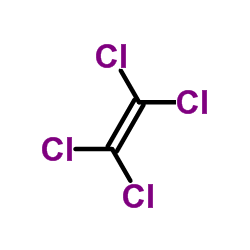 CAS#:127-18-4
CAS#:127-18-4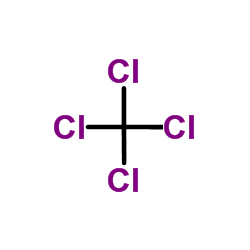 CAS#:56-23-5
CAS#:56-23-5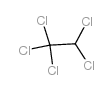 CAS#:76-01-7
CAS#:76-01-7 CAS#:67-72-1
CAS#:67-72-1 CAS#:16650-10-5
CAS#:16650-10-5 CAS#:98-07-7
CAS#:98-07-7 CAS#:79-04-9
CAS#:79-04-9 CAS#:75-44-5
CAS#:75-44-5 CAS#:37021-34-4
CAS#:37021-34-4 CAS#:110655-92-0
CAS#:110655-92-0 CAS#:35302-72-8
CAS#:35302-72-8 CAS#:3702-98-5
CAS#:3702-98-5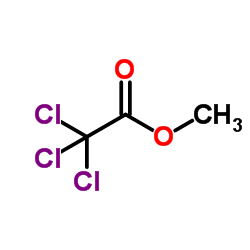 CAS#:598-99-2
CAS#:598-99-2 CAS#:23170-77-6
CAS#:23170-77-6 CAS#:310886-79-4
CAS#:310886-79-4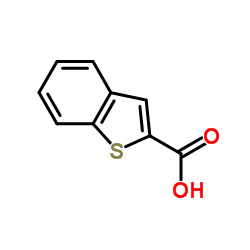 CAS#:6314-28-9
CAS#:6314-28-9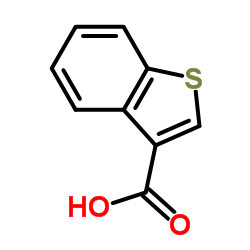 CAS#:5381-25-9
CAS#:5381-25-9![2-[(2,2,2-trichloroacetyl)amino]acetic acid structure](https://image.chemsrc.com/caspic/430/15166-50-4.png) CAS#:15166-50-4
CAS#:15166-50-4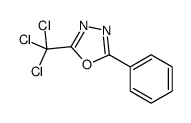 CAS#:1456-20-8
CAS#:1456-20-8
