Biapenem
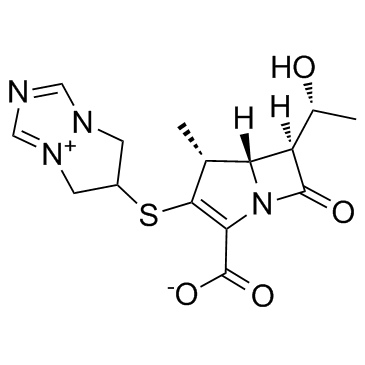
Biapenem structure
|
Common Name | Biapenem | ||
|---|---|---|---|---|
| CAS Number | 120410-24-4 | Molecular Weight | 350.393 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H18N4O4S | Melting Point | 265-271°C (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of BiapenemBiapenem is a parenteral carbapenem antibacterial agent with a broad spectrum.Target: AntibacterialBiapenem is a carbapenem antibiotic of in vitro antibacterial activity encompassing many Gramnegative and Gram-positive aerobic and anaerobic bacteria, including species producing β-lactamases. Biapenem is more stable than imipenem, mero-penem and panipenem to hydrolysis by human renal dihydropeptidase-I (DHP-I), and therefore does not require the coadministration of a DHP-I inhibitor. In randomised, nonblind or double-blind clinical trials, biapenem showed good clinical and bacteriological efficacy (similar to that of imipenem/ cilastatin) in the treatment of adult patients with intra-abdominal infections, lower respiratory infections or complicated urinary tract infections. |
| Name | biapenem |
|---|---|
| Synonym | More Synonyms |
| Description | Biapenem is a parenteral carbapenem antibacterial agent with a broad spectrum.Target: AntibacterialBiapenem is a carbapenem antibiotic of in vitro antibacterial activity encompassing many Gramnegative and Gram-positive aerobic and anaerobic bacteria, including species producing β-lactamases. Biapenem is more stable than imipenem, mero-penem and panipenem to hydrolysis by human renal dihydropeptidase-I (DHP-I), and therefore does not require the coadministration of a DHP-I inhibitor. In randomised, nonblind or double-blind clinical trials, biapenem showed good clinical and bacteriological efficacy (similar to that of imipenem/ cilastatin) in the treatment of adult patients with intra-abdominal infections, lower respiratory infections or complicated urinary tract infections. |
|---|---|
| Related Catalog | |
| References |
[1]. Perry, C.M. and T. Ibbotson, Biapenem. Drugs, 2002. 62(15): p. 2221-34; discussion 2235. |
| Melting Point | 265-271°C (dec.) |
|---|---|
| Molecular Formula | C15H18N4O4S |
| Molecular Weight | 350.393 |
| Exact Mass | 350.104889 |
| PSA | 127.67000 |
| Index of Refraction | 1.651 |
| InChIKey | MRMBZHPJVKCOMA-YJFSRANCSA-N |
| SMILES | CC(O)C1C(=O)N2C(C(=O)[O-])=C(SC3Cn4cnc[n+]4C3)C(C)C12 |
| Storage condition | -20?C Freezer |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
Biapenem CAS#:120410-24-4 |
| Literature: Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 - 4249 |
|
~% 
Biapenem CAS#:120410-24-4 |
| Literature: Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 - 4249 |
|
~% 
Biapenem CAS#:120410-24-4 |
| Literature: Journal of Organic Chemistry, , vol. 57, # 15 p. 4243 - 4249 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2010: General view of the pathogens' antibacterial susceptibility.
J. Infect. Chemother. 21 , 410-20, (2015) The nationwide surveillance on antimicrobial susceptibility of bacterial respiratory pathogens from patients in Japan, was conducted by Japanese Society of Chemotherapy, Japanese Association for Infec... |
|
|
Novel approach to optimize synergistic carbapenem-aminoglycoside combinations against carbapenem-resistant Acinetobacter baumannii.
Antimicrob. Agents Chemother. 59 , 2286-98, (2015) Acinetobacter baumannii is among the most dangerous pathogens and emergence of resistance is highly problematic. Our objective was to identify and rationally optimize β-lactam-plus-aminoglycoside comb... |
|
|
Biapenem inactivation by B2 metallo β-lactamases: energy landscape of the post-hydrolysis reactions.
PLoS ONE 7(1) , e30079, (2012) The first line of defense by bacteria against β-lactam antibiotics is the expression of β-lactamases, which cleave the amide bond of the β-lactam ring. In the reaction of biapenem inactivation by B2 m... |
| Biapenem (JAN/USAN) |
| EINECS 204-352-8 |
| 5H-Pyrazolo[1,2-a][1,2,4]triazol-4-ium, 6-[[(4R,5S,6S)-2-carboxy-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-, inner salt |
| BiapeneM Crude |
| Biapenem [USAN:INN] |
| (4R,5S,6S)-3-(6,7-Dihydro-5H-pyrazolo[1,2-a][1,2,4]triazol-4-ium-6-ylsulfanyl)-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate |
| [4R-[4a,5b,6b(R*)]]-6-[[2-Carboxy-6-(1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]hept-2-en-3-yl]thio]-6,7-dihydro-5 H-pyrazolo[1,2-a][1,2,4]triazol-4-ium inner salt |
| LJ C10627 |
| omegacin |
| MFCD00864863 |
| Biapenem |
| LJ C10,627 |
| Biapenern |
| L-627 |

![4-nitrobenzyl (4R,5R,6S)-6-[(1R)-1-hydroxyethyl]-4-methyl-3,7-dioxo-1-azabicyclo[3.2.0]-heptane-2-carboxylate structure](https://image.chemsrc.com/caspic/055/104873-15-6.png)
![p-Nitrobenzyl (1R,5S,6S)-2-[[(N,N-Bis(p-nitrobenzyloxycarbonyl)pyrazolidin-4-yl]thio]-6-[(1R)-1-hydroxyethyl]-1-methylcarbapen-2-em-3-carboxylate structure](https://image.chemsrc.com/caspic/071/120764-68-3.png)

