55079-83-9
| Name | all-trans-acitretin |
|---|---|
| Synonyms |
13-cis Acitretin
retinoidetretin Acetretin ro10-1670 SORIATANE etretin (2Z,4E,6E,8E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid Acitreti (2Z,4E,6E,8E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid Acitretin Acritretin all-trans-etretin EINECS 259-474-4 MFCD00866632 13-cis-Acitretin NEOTIGASON |
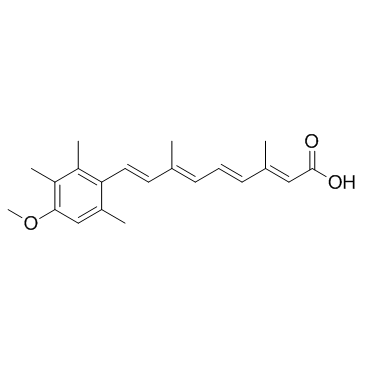
| Description | Acitretin(Ro 10-1670) is a second-generation, systemic retinoid that has been used in the treatment of psoriasis.Target: RAR/RXRAcitretin is a second-generation, systemic retinoid that has been approved for the treatment of psoriasis since 1997. It can be considered one of the treatments of choice for pustular and erythrodermic psoriasis. However, the efficacy of acitretin as a monotherapy for plaque psoriasis is less, although it is often used in combination therapy with other systemic psoriasis therapies, especially ultraviolet B or psoralen plus ultraviolet A phototherapy, to increase efficacy. Such combination treatments may potentially minimise toxicity by using lower doses of each of the two agent [1].Thirty-nine male adult Wistar albino rats were divided into 3 groups as two experimental groups and one control group. The first group consisting 14 rats were applied orally standard dose (0.75 mg/kg/day) acitretin and the second group consisting 16 rats were applied high dose (1.5 mg/kg/day) acitretin.Acitretin was given within dimetil sulphoxide (DMSO), which was diluted with saline solution as a ratio of 1/10, in order to increase its solubility. The control group consisting 9 rats were given only saline solution including DMSO for 8 weeks. After 8 weeks of the administration, half of the rats in the first and second groups and the entire control group were sacrificed under deep ether anaesthesia and bilateral orchiectomy was made. The remainingrats were compared with the control group using a similar method at the end of 8 weeks of wash-off period. The orchiectomy materials were histopathologically evaluated under the light microscope for spermatogenesis according to parameters including spermatogenetic activity, spermatogenetic organization, seminiferous tubular diameter, interstitial Leydig cells and fibroblasts. In our study it was concluded that the standard and high doses of acitretin do not have any effect on the spermatogenesis of threats [2].Clinical indications: PsoriasisFDA Approved Date: Toxicity: nausea; headache; itching; red or flaky skin; dry or red eyes; dry mouth; depression; 0cystitis acne or hair loss |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 521.3±38.0 °C at 760 mmHg |
| Melting Point | 228-230ºC |
| Molecular Formula | C21H26O3 |
| Molecular Weight | 326.429 |
| Flash Point | 180.3±20.3 °C |
| Exact Mass | 326.188202 |
| PSA | 46.53000 |
| LogP | 5.73 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.570 |
| Storage condition | 2-8°C |
| Stability | LIGHT SENSITIVE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H319-H360-H410 |
| Precautionary Statements | P201-P273-P305 + P351 + P338-P308 + P313-P501 |
| Hazard Codes | T:Toxic |
| Risk Phrases | R36/38;R61;R50/53 |
| Safety Phrases | S53-S37/39-S45-S60-S61 |
| RIDADR | UN 3077 |
| WGK Germany | 3 |
| RTECS | RA8460000 |
| Packaging Group | III |
| Hazard Class | 9.0 |