590-63-6
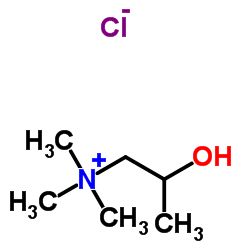
| Name | bethanechol chloride |
|---|---|
| Synonyms |
Methacholinium chloride
Besacholine Mechothane 1-propanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride Methacholine chloride β-Methylcholine chloride urethan Urecholine chloride Uro-Carb 2-carbamoyloxypropyl(trimethyl)azanium,chloride b-Methylacetylcholine Chloride Acetyl-b-methylcholine Chloride 2-Acetoxy-N,N,N-trimethylpropan-1-aminium chloride (2-Carbamoyloxy-propyl)-trimethyl-ammonium,Chlorid 2-(Carbamoyloxy)-N,N,N-trimethylpropan-1-aminium chloride Carbamate of (2-Hydroxypropyl)trimethylammonium Chloride Mictone 2-(acetyloxy)-N,N,N-trimethylpropan-1-aminium chloride 2-(Carbamoyloxy)-N,N,N-trimethyl-1-propanaminium chloride 2-[(aminocarbonyl)oxy]-N,N,N-trimethylpropan-1-aminium chloride Carbamylmethylcholine chloride Urecholine 2-[(Aminocarbonyl)oxy]-N,N,N-trimethyl-1-propanaminium Chloride (2-Carbamoyloxypropyl)trimethylammonium chloride Bethanechol chloride (2-carbamoyloxy-propyl)-trimethyl-ammonium,chloride Trimethyl-b-acetoxypropylammonium Chloride Mechotane O-Acetyl-b-methylcholine Chloride BTC Mecothane 2-carbamyloxy-1-(N,N,N-trimethyl)-propylammonium chloride MFCD00055224 1-Propanaminium, 2-[(aminocarbonyl)oxy]-N,N,N-trimethyl-, chloride (1:1) methylcarbachol chloride UNII:0W5ETF9M2K EINECS 209-686-8 Bethanechol 1-Propanaminium, 2-(acetyloxy)-N,N,N-trimethyl-, chloride (1:1) DUVOID Myocholine 2-Acetoxy-N,N,N-trimethyl-1-propanaminium chloride (±)-Acetyl-b-methylcholine Chloride Bethanechol (chloride) |
| Description | Bethanechol Chloride is a selective muscarinic receptor agonist without any effect on nicotinic receptors.Target: mAChRBethanechol is a parasympathomimetic choline carbamate that selectively stimulates muscarinic receptors without any effect on nicotinic receptors. Unlike acetylcholine, bethanechol is not hydrolyzed by cholinesterase and will therefore have a long duration of action. Oral bethanechol significantly improves contraction pressures and bolus transit in the smooth muscle portion of the esophagus in patients with severe IEM [1]. Bethanechol has potential benefit in the treatment of cerebral palsy [2]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 187-190ºC |
|---|---|
| Molecular Formula | C7H17ClN2O2 |
| Molecular Weight | 196.68 |
| PSA | 52.32000 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: 1.7 g/mL stable for several days at 4°C. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | BR5425000 |
| HS Code | 2924199090 |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |