7279-75-6
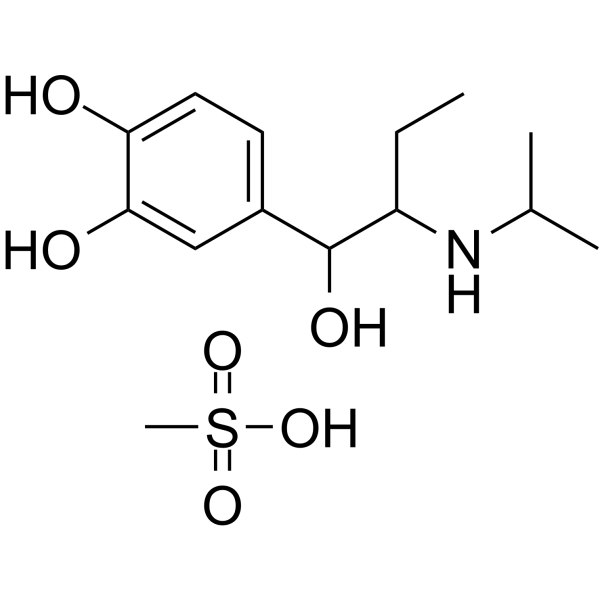
| Name | 4-[1-hydroxy-2-(propan-2-ylamino)butyl]benzene-1,2-diol,methanesulfonic acid |
|---|---|
| Synonyms |
Isoetharine mesylate salt
4-[1-Hydroxy-2-[(1-methylethyl)amino]butyl]-1,2-benzenediol mesylate salt EINECS 230-695-8 1,2-Benzenediol,4-(1-hydroxy-2-((1-methylethyl)amino)-butyl)-,methanesulfonate (salt) Isotharine mesylate ISOETHARINE MESYLATE Isoetharine mesylate (USP) Isoetarine mesilate |
| Description | Isoetharine (Isoetarine) mesylate is an orally active selective agonist of β-adrenergic receptors. Isoetharine mesylate is a catechol-like drug and catechol O-methyltransferase (COMT) mediates its methylation. Isoetharine mesylate can promote the production of cAMP which stimulates the relaxation of smooth muscle cells and can be used as an emphysema, bronchitis and bronchodilator[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Isoetharine mesylate (50 μM, 18 hours) can induce the production and release of [35S]sulfated metabolites of catecholic drugs in HepG2 human hepatoma cells[1]. |
| In Vivo | Isoetharine mesylate inhibits melanin deposition with the AC50 value of 5.10 mM and complete inhibition of pigment production at 7.50 mM in the zebrafish larvae model[2]. |
| References |
| Boiling Point | 429.3ºC at 760 mmHg |
|---|---|
| Molecular Formula | C14H25NO6S |
| Molecular Weight | 335.41600 |
| Flash Point | 156.7ºC |
| Exact Mass | 335.14000 |
| PSA | 135.47000 |
| LogP | 2.88350 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22 |
| Safety Phrases | 36 |
| RIDADR | NONH for all modes of transport |