CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KR5919600
-
CHEMICAL NAME :
-
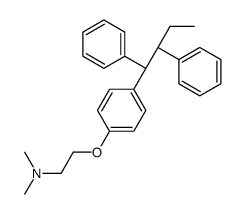
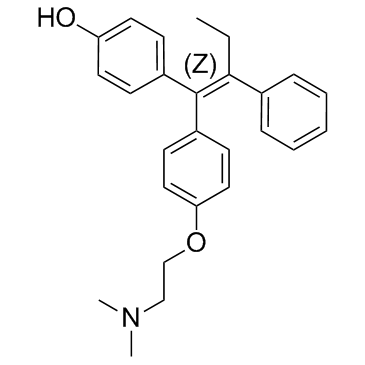
Ethylamine, N,N-dimethyl-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-, (Z)-
-
CAS REGISTRY NUMBER :
-
10540-29-1
-
LAST UPDATED :
-
199801
-
DATA ITEMS CITED :
-
49
-
MOLECULAR FORMULA :
-
C26-H29-N-O
-
MOLECULAR WEIGHT :
-
371.56
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
5600 ug/kg/1W-I
-
TOXIC EFFECTS :
-
Skin and Appendages - breast
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
200 ug/kg/D
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting Blood - leukopenia Blood - thrombocytopenia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
5600 ug/kg/2W-I
-
TOXIC EFFECTS :
-
Blood - normocytic anemia Musculoskeletal - joints
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4100 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2150 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
575 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
330 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases Biochemical - Metabolism (Intermediary) - other proteins
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
1001 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases Biochemical - Metabolism (Intermediary) - other proteins
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6825 mg/kg/65W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Liver - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
lactating female 5 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - postpartum
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2250 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 9-11 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Effects on Embryo or Fetus - other effects to embryo
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
50 ug/kg
-
SEX/DURATION :
-
female 4 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2250 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
100 ug/kg
-
SEX/DURATION :
-
female 3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
75 ug/kg
-
SEX/DURATION :
-
female 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
DOSE :
-
3500 ug/kg
-
SEX/DURATION :
-
female 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Maternal Effects - menstrual cycle changes or disorders Reproductive - Maternal Effects - other effects
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2400 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - oogenesis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
50 ug/kg
-
SEX/DURATION :
-
female 3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
7200 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
25 mg/kg
-
SEX/DURATION :
-
female 5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3250 ug/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
Morphological transformation
-
TYPE OF TEST :
-
DNA adduct
-
TYPE OF TEST :
-
DNA adduct
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TYPE OF TEST :
-
DNA inhibition
-
TYPE OF TEST :
-
Cytogenetic analysis
-
TYPE OF TEST :
-
Sex chromosome loss and nondisjunction
-
TYPE OF TEST :
-
Mutation in mammalian somatic cells
-
TYPE OF TEST :
-
DNA adduct
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
480 umol/kg/4D (Continuous)
-
REFERENCE :
-
CRNGDP Carcinogenesis (London). (Oxford Univ. Press, Pinkhill House, Southfield Road, Eynsham, Oxford OX8 1JJ, UK) V.1- 1980- Volume(issue)/page/year: 13,2197,1992 *** REVIEWS *** IARC Cancer Review:Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,253,1996 IARC Cancer Review:Human Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,253,1996 IARC Cancer Review:Group 1 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 66,253,1996 TOXICOLOGY REVIEW JTEHD6 Journal of Toxicology and Environmental Health. (Hemisphere Pub., 1025 Vermont Ave., NW, Washington, DC 20005) V.1- 1975/76- Volume(issue)/page/year: 4,363,1978 TOXICOLOGY REVIEW CLECAP Clinical Endocrinology (Oxford). (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1972- Volume(issue)/page/year: 4,551,1975 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X7229 No. of Facilities: 9 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 339 (estimated) No. of Female Employees: 170 (estimated)
|




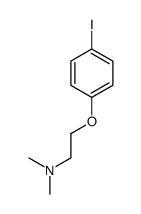
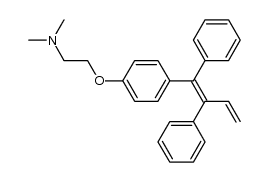
![[4-[2-(dimethylamino)ethoxy]phenyl]boronic acid structure](https://image.chemsrc.com/caspic/458/194594-60-0.png)
![2-[4-[(E)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine structure](https://image.chemsrc.com/caspic/227/13002-65-8.png)
![2-[4-[(Z)-1,2-diphenylbut-1-enyl]-2-iodophenoxy]-N,N-dimethylethanamine structure](https://image.chemsrc.com/caspic/010/76070-10-5.png)