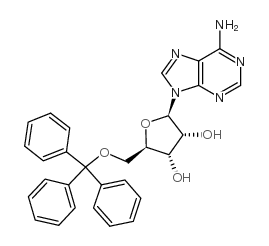
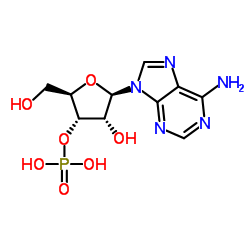
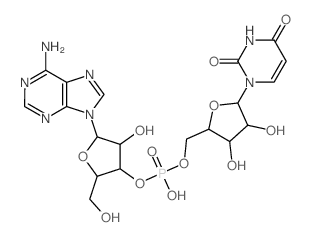
130-49-4
| Name | adenosine 2'-phosphate |
|---|---|
| Synonyms |
Adenosine Monophosphate Hydrate (2'- and 3'- mixture) from Yeast
MFCD00151215 Adenosine 2'-monophosphate Adenylic Acid Hydrate (2'- and 3'- mixture) from Yeast Methionyl adenylate adenosine 2'-monophosphoric acid Adenosine 2'(3')-monophosphate mixed isomers Adenosine 2'-phosphate 2'(3')-AMP Hydrate (2'- and 3'- mixture) from Yeast Methioninyl adenylate 2'-Adenylic acid EINECS 204-990-7 L-METHIONYL ADENYLATE 2'-adenosine monophosphate |
| Description | Adenosine-2'-monophosphate (2'-AMP) is converted by extracellular 2’,3'-CAMP. Adenosine-2'-monophosphate is further metabolized to extracellular adenosine (a mechanism called the extracellular 2’,3’-cAMP-adenosine pathway). Adenosine-2'-monophosphate inhibits LPS-induced TNF-α and CXCL10 production via A2A receptor activation[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite A2A adenosine receptor A2B adenosine receptor |
| In Vitro | Adenosine-2'-monophosphate (2'-AMP) (0-100 µM; daily for 4 days) inhibits proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors[1]. Adenosine-2'-monophosphate (30 μM; 24 hours) inhibits LPS induced (100 ng/ml) TNF-α and CXCL10 production in primary murine microglia[1]. |
| References |
| Density | 2.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 815.5±75.0 °C at 760 mmHg |
| Molecular Formula | C10H14N5O7P |
| Molecular Weight | 347.221 |
| Flash Point | 447.0±37.1 °C |
| Exact Mass | 347.063080 |
| PSA | 195.88000 |
| LogP | -1.74 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.905 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| WGK Germany | 3 |
| RTECS | AU7480300 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |


![[5-(4-amino-2-oxo-pyrimidin-1-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[5-(6-aminopurin-9-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxy-phosphinic acid structure](https://image.chemsrc.com/caspic/177/4833-63-0.png)
![[5-(2-amino-6-oxo-3H-purin-9-yl)-3,4-dihydroxy-oxolan-2-yl]methoxy-[5-(6-aminopurin-9-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxy-phosphinic acid structure](https://image.chemsrc.com/caspic/314/3352-23-6.png)