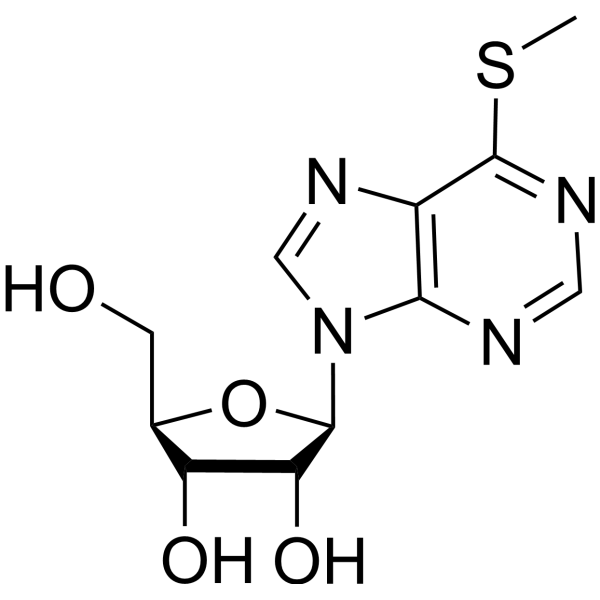
58-61-7
| Name | adenosine |
|---|---|
| Synonyms |
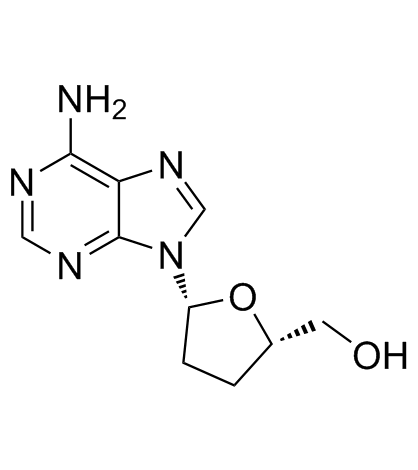
(2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydrofuran-3,4-diol
Adenosin 9β-D-ribofuranosyl-9H-Purin-6-amine Adesine 1-(6-amino-9H-purin-9-yl)-1-deoxy-β-D-Ribofuranose 6-Amino-9-b-D-ribofuranosyl-9H-purine Adenocor Sandesin (2R,3R,4S,5R)-2-(6-Amino-9H-purin-9-yl)-5-(hydroxymethyl)tetrahydro-3,4-furandiol 9-β-δ-Ribofuranosidoadenine (-)-adenosine 9-β-δ-Ribofuranosyl-9H-purin-6-amine (–)-adenosine 9-b-D-Ribofuranosidoadenine D-Adenosine MFCD00005752 Adenosine Adenine-9-beta-D-ribofuranoside Adrekar 9-β-D-Ribofuranosyladenine 9-beta-D-Ribofuranosyladenine 9-b-D-Ribofuranosyl-9H-purin-6-amine Boniton Adenine riboside Adenoscan adenine-9β-D-Ribofuranoside Adenocard EINECS 200-389-9 9-β-D-Arabinofuranosyladenine Myocol |
| Description | Adenosine is a nucleoside composed of a molecule of adenine attached to a ribose sugar molecule (ribofuranose) moiety via a β-N9-glycosidic bond.Target: Nucleoside antimetabolite/analogAdenosine plays an important role in biochemical processes, such as energy transfer — as adenosine triphosphate (ATP) and adenosine diphosphate (ADP) — as well as in signal transduction as cyclic adenosine monophosphate, cAMP. It is also an inhibitory neurotransmitter, believed to play a role in promoting sleep and suppressing arousal. Adenosine also plays a role in regulation of blood flow to various organs through vasodilation.Adenosine is an endogenous purine nucleoside that modulates many physiological processes. Cellular signaling by adenosine occurs through four known adenosine receptor subtypes. Extracellular adenosine concentrations from normal cells are approximately 300 nM; however, in response to cellular damage (e.g. in inflammatory or ischemic tissue), these concentrations are quickly elevated (600–1,200 nM). Thus, in regard to stress or injury, the function of adenosine is primarily that of cytoprotection preventing tissue damage during instances of hypoxia, ischemia, and seizure activity. Activation of A2A receptors produces a constellation of responses that in general can be classified as anti-inflammatory. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 2.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 676.3±65.0 °C at 760 mmHg |
| Melting Point | 234-236ºC |
| Molecular Formula | C10H13N5O4 |
| Molecular Weight | 267.241 |
| Flash Point | 362.8±34.3 °C |
| Exact Mass | 267.096741 |
| PSA | 139.54000 |
| LogP | -1.02 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.907 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AU7175000 |
| HS Code | 2934999090 |
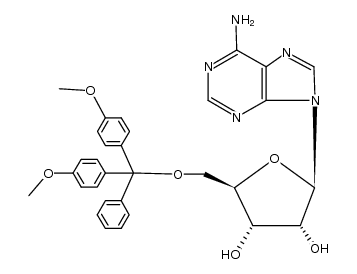
| Precursor 9 | |
|---|---|
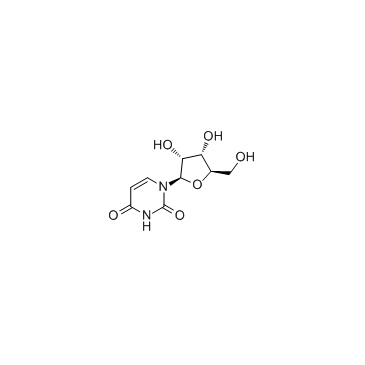
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |





![[(2S,4R,5R)-5-(6-aminopurin-9-yl)-4-azidooxolan-2-yl]methanol structure](https://image.chemsrc.com/caspic/130/110142-99-9.png)