77883-43-3
| Name | doxazosin mesylate |
|---|---|
| Synonyms |
piperazine, 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(2,3-dihydro-1,4-benzodioxin-2-yl)carbonyl]-, methanesulfonate (1:1)
Normothen [4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](2,3-dihydro-1,4-benzodioxin-2-yl)methanone methanesulfonate [4-(4-Amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl](2,3-dihydro-1,4-benzodioxin-2-yl)methanone methanesulfonate (1:1) Doxazosin mesylate Hydrogen methanesulfonate - [4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)methanone (1:1:1) Cardura MFCD00216023 Methanone, [4-(4-amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)-, methanesulfonate (1:1) UNII:86P6PQK0MU [4-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-1-piperazinyl](2,3-dihydro-1,4-benzodioxin-2-yl)methanone methanesulfonate [4-(4-amino-6,7-dimethoxyquinazolin-2-yl)piperazin-1-yl]-(2,3-dihydro-1,4-benzodioxin-3-yl)methanone,methanesulfonic acid Doxazosin (mesylate) |
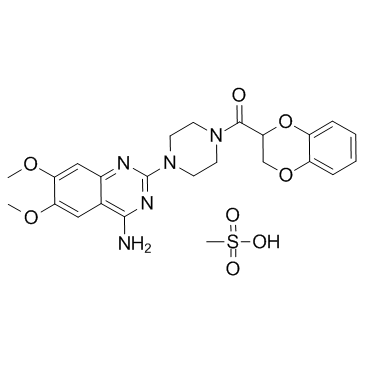
| Description | Doxazosin mesylate(UK 33274) is a quinazoline-derivative that selectively antagonizes postsynaptic α1-adrenergic receptors.Target: α1-adrenergic receptorDoxazosin (mesylate) is the mesylate salt form of doxazosin, which is a long-lasting inhibitor of α1-adrenoceptors that is widely used to treat benign prostatic hyperplasia and lower urinary tract symptoms [1]. doxazosin may have a direct inhibitory effect on cholesterol synthesis independent of the LDL receptor. The inhibition of cholesterol synthesis by doxazosin may cause cells to compensate by upregulating the LDL receptor, thereby increasing the importation of lipoprotein cholesterol and reducing LDL cholesterol in the medium [2]. Doxazosin monotherapy was effective in eight of 12 patients (66.7%), and combined therapy with a beta-blocker was effective in 11 of 12 patients (91.7%). The mean pulse rate remained constant throughout therapy. Adverse reactions were minor and transient and occurred in only three patients. Urinary and plasma catecholamine levels tended to decrease or remained unchanged during doxazosin therapy [3]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 718ºC at 760 mmHg |
|---|---|
| Melting Point | 275-277ºC |
| Molecular Formula | C24H29N5O8S |
| Molecular Weight | 547.581 |
| Flash Point | 388ºC |
| Exact Mass | 547.173706 |
| PSA | 175.02000 |
| LogP | 2.88670 |
| Storage condition | Desiccate at RT |
| Stability | Protect from light |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | TK8044000 |