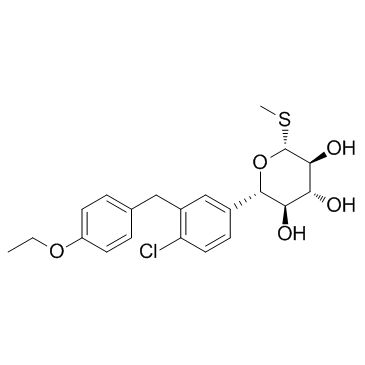
1018899-04-1
| Name | (2S,3R,4R,5S,6R)-2-[4-chloro-3-[(4-ethoxyphenyl)methyl]phenyl]-6-methylsulfanyloxane-3,4,5-triol |
|---|---|
| Synonyms |
sotagliflozin
LP-802034 (2S,3R,4R,5S,6R)-2-[4-chloro-3-(4-ethoxy-benzyl)-phenyl]-6-methylsulfanyl-tetrahydro-pyran-3,4,5-triol LX-4211 (2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(methylthio)tetrahydro-2H-pyran-3,4,5-triol LX4211 CS-1069 LX 4211 Methyl (5S)-5-[4-chloro-3-(4-ethoxybenzyl)phenyl]-1-thio-β-L-xylopyranoside UNII-6B4ZBS263Y |
| Description | LX-4211 is a potent dual SGLT2/1 inhibitor; Antidiabetic agents.IC50 value:Target: SGLT1/2LX4211 enhanced urinary glucose excretion by inhibiting SGLT2-mediated renal glucose reabsorption; markedly and significantly improved multiple measures of glycemic control, including fasting plasma glucose, oral glucose tolerance, and HbA(1c); and significantly lowered serum triglycerides. LX4211 also mediated trends for lower weight, lower blood pressure, and higher glucagon-like peptide-1 levels. In a follow-up single-dose study in 12 patients with T2DM, LX4211 (300 mg) significantly increased glucagon-like peptide-1 and peptide YY levels relative to pretreatment values, probably by delaying SGLT1-mediated intestinal glucose absorption [1]. LX4211-treated mice and SGLT1-/- mice also had increased GLP-1 AUC values, decreased glucose-dependent insulinotropic polypeptide (GIP) AUC values, and decreased blood glucose excursions during the 6 hours after a challenge with oral glucose alone [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 607.9±55.0 °C at 760 mmHg |
| Molecular Formula | C21H25ClO5S |
| Molecular Weight | 424.938 |
| Flash Point | 321.4±31.5 °C |
| Exact Mass | 424.111115 |
| PSA | 104.45000 |
| LogP | 5.63 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.642 |
| Storage condition | -20℃ |