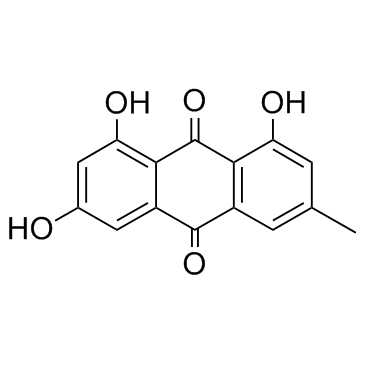
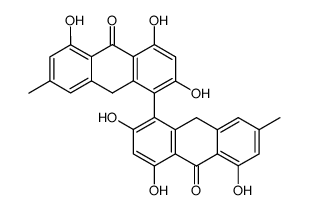
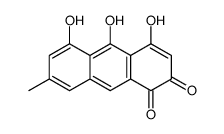
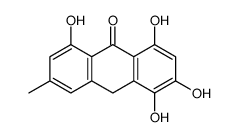
491-60-1
| Name | 1,3,8-trihydroxy-6-methyl-10H-anthracen-9-one |
|---|---|
| Synonyms |
1,3,8-Trihydroxy-6-methyl-anthron
6-methyl-1,3,8-trihydroxy-10H-anthracene-9-one Emodinanthrone 1,3,8-trihydroxy-6-methyl-anthrone |
| Description | Emodinanthrone, an anthraquinone, is a sprecursor of Emodin with antibiotic activity. Emodinanthrone inhibits respiration-driven solute transport at micromolar concentrations in membrane vesicles of Escherichia coli[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Emodinanthrone is used as an alternative substrate for the AknX oxygenase assay[1]. |
| References |
| Density | 1.471 g/cm3 |
|---|---|
| Boiling Point | 556.2ºC at 760 mmHg |
| Melting Point | 254-258 °C(dec.) |
| Molecular Formula | C15H12O4 |
| Molecular Weight | 256.25300 |
| Exact Mass | 256.07400 |
| PSA | 77.76000 |
| LogP | 2.24700 |
| Vapour Pressure | 5.6E-13mmHg at 25°C |
| Index of Refraction | 1.723 |
| Hazard Codes | Xi |
|---|
|
~96% 
491-60-1 |
| Literature: HY BIOPHARMA, INC.; TOBIA, Alfonso, J.; CABANA, Bernard, E.; VADLAPATLA, Venkata; CONNOLLY, Ronald, H. Patent: WO2011/34922 A1, 2011 ; Location in patent: Page/Page column 9 ; |
|
~87% 
491-60-1 |
| Literature: Liang, Jing Lu; Cha, Hyo Chang; Lee, Seung Ho; Son, Jong-Keun; Chang, Hyeun Wook; Eom, Ji-Eun; Kwon, Youngjoo; Jahng, Yurngdong Archives of Pharmacal Research, 2012 , vol. 35, # 3 p. 447 - 454 |
|
~% 
491-60-1 |
| Literature: Liang, Jing Lu; Cha, Hyo Chang; Lee, Seung Ho; Son, Jong-Keun; Chang, Hyeun Wook; Eom, Ji-Eun; Kwon, Youngjoo; Jahng, Yurngdong Archives of Pharmacal Research, 2012 , vol. 35, # 3 p. 447 - 454 |
|
~% 
491-60-1 |
| Literature: Liang, Jing Lu; Cha, Hyo Chang; Lee, Seung Ho; Son, Jong-Keun; Chang, Hyeun Wook; Eom, Ji-Eun; Kwon, Youngjoo; Jahng, Yurngdong Archives of Pharmacal Research, 2012 , vol. 35, # 3 p. 447 - 454 |
|
~% 
491-60-1 |
| Literature: Anslow; Breen; Raistrick Biochemical Journal, 1940 , vol. 34, p. 159,165 |
|
~% 
491-60-1 |
| Literature: Oxford; Raistrick Biochemical Journal, 1940 , vol. 34, p. 790,799 |
|
~% 
491-60-1 |
| Literature: Anslow; Breen; Raistrick Biochemical Journal, 1940 , vol. 34, p. 159,165 |
|
~% 
491-60-1 |
| Literature: Eder Archiv der Pharmazie (Weinheim, Germany), 1915 , vol. 253, p. 32 Archiv der Pharmazie (Weinheim, Germany), 1916 , vol. 254, p. 18,30 Full Text Show Details Tutin; Clewer Journal of the Chemical Society, 1912 , vol. 101, p. 294 |
|
~% 
491-60-1 |
| Literature: Liang, Jing Lu; Cha, Hyo Chang; Lee, Seung Ho; Son, Jong-Keun; Chang, Hyeun Wook; Eom, Ji-Eun; Kwon, Youngjoo; Jahng, Yurngdong Archives of Pharmacal Research, 2012 , vol. 35, # 3 p. 447 - 454 |
| Precursor 7 | |
|---|---|
| DownStream 5 | |