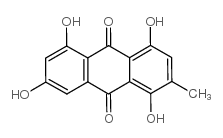
518-82-1
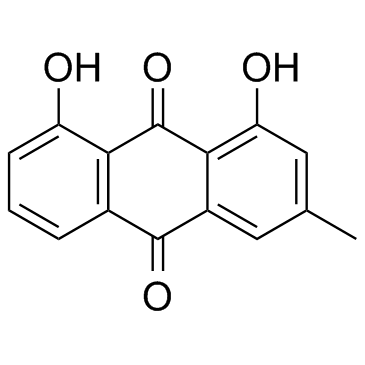
| Name | emodin |
|---|---|
| Synonyms |
6-Methyl-1,3,8-trihydroxyanthraquinone
MFCD00001207 Ecdyson 1,3,8-Trihydroxy-6-methylanthracene-9,10-dione Alatinone 1,3,8-Trihydroxy-6-methylanthraquinone EMODINE 1,3,8-Tri-hydroxy-6-methyl-anthra-quinone Emodin EMODIN 99 1,3,8-trihydroxy-6-methyl-9,10-anthraquinone EINECS 208-258-8 EMODOL Archin schuttgelb Emdin |
| Description | Emodin is a broad-spectrum anticancer agent. Emodin inhibits casein kinase II (CKII) activity with IC50 of 2 μM. |
|---|---|
| Related Catalog | |
| Target |
CKII:2 μM (IC50) |
| In Vitro | Emodin, an anthraquinone derivative, selectively inhibits casein kinase II(CKII), a Ser/Thr kinase, as a competitive inhibitor. Emodin inhibits CKII activity with IC50 of 2 μM, which is two to three orders of magnitude lower than those against the other kinases. Enzyme kinetic assays show that Emodin inhibits CKII activity as acompetitive inhibitor against ATP with Ki of 7.2 μM[1]. Emodin is a broad-spectrum inhibitory agent of cancer cells, including leukemia, lung cancer, human tongue squamous cancer, colon cancer, gallbladder cancer, pancreatic cancer, breast cancer, human cervical cancer and hepatic carcinoma cells. Emodin inhibits A549, HepG2, OVCAR-3, HeLa and Madin-Darby Canine Kidney (MDCK) cells with IC50 of 19.54, 12.79, 25.82, 12.14 and 5.81 μg/mL. The anticancer mechanisms of Emodin are involved in many biological pathways, such as casein kinase II and ERK1/2[2]. Emodin is applied as a Reactive oxygen species (ROS) generator in combination with cisplatin in T24 and J82 human bladder cancer cells. Emodin kills T24 and J82 cells in the dose-dependent and time-dependent manner, and it is less toxic to HCV-29 cells. The concentration of 20 and 15 μM is selected as appropriate doses for investigating chemotherapeutic sensitivity of T24 and J82 cells at 24 h, respectively[3]. |
| In Vivo | Mice treated with Emodin (50 mg/kg) and Cisplatin (1 mg/kg) have significantly smaller tumors than those from the other groups. In addition, no notable differences on the body weight loss are observed among groups and no obvious necrosis and abnormity are observed in the sections of liver, kidney and heart. These results demonstrate that Emodin/cisplatin co-treatment can significantly suppress tumor growth in vivo with no distinct side effects. Consistent with in vitro experiment, TUNEL assay shows that Emodin/cisplatin combination significantly increased cell apoptosis in xenograft tumors. Emodin/Cisplatin co-treatment group also has lower MRP1 expression than the other groups[3]. |
| Cell Assay | The T24 human bladder cancer cells, the HCV-29 normal bladder epithelial cells and J82 human bladder cancer cells are are cultured in RPMI 1640 medium supplemented with 10 % fetal bovine serum at 37°C in a humidified atmosphere containing 5 % CO2. Cells are seeded in 96-well plates with 2×104 cells per well. The cells are incubated with Emodin for 24 h at different concentrations (0, 5, 10, 20, 30, 40, 50, 60, 70 μM) and chose the critical concentration (20 μM) treated with cells for 0, 6, 12, 24, 48, 72, 96 h. The cells are incubated with cisplatin for 24 h at different concentrations (0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0 μg/mL). MTT assay is used to analyze the cell viability. Cells are treated with drugs for 24 h and apoptotic rates are assessed with flow cytometry using AnnexinV-fluorescein isothiocyanate (AnnexinV-FITC)/propidium iodide (PI) kit. Samples are prepared according to the manufacturer’s instruction and analyzed by a flow cytometry (FCM) Calibur[3]. |
| Animal Admin | Mice[3] 3×106 T24 cells are harvested, washed, and resuspended in serum-free optimum medium and then injected subcutaneously into 6-week old BALB/c-nu/nu mice (n=8 mice per group). Three days after inoculation, the mice are intraperitoneally administered with PBS, Emodin (50 mg/kg), Cisplatin (1 mg/kg), or Emodin/cisplatin every two days. On day 18, every mouse is sacrificed. After body weight measurement, tumors are isolated, weighted and fixed in 4 % paraformaldehyde (PFA). Hearts, livers and kidneys are stained with Hematoxylin & Eosin to determine the systemic toxicity. Terminal deoxynucleotidyl transferase(TdT)-mediated dUTP nick end label (TUNEL) assay is performed on paraformaldehyde-fixed and paraffin-embedded tumor sections. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.9±39.0 °C at 760 mmHg |
| Melting Point | 255 °C (dec.)(lit.) |
| Molecular Formula | C15H10O5 |
| Molecular Weight | 270.237 |
| Flash Point | 322.8±23.6 °C |
| Exact Mass | 270.052826 |
| PSA | 94.83000 |
| LogP | 5.03 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.745 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble | <0.1 g/100 mL at 19 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CB7920600 |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914690090 |
|---|---|
| Summary | 2914690090 other quinones。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |




![[4,5-diacetyloxy-7-(bromomethyl)-9,10-dioxoanthracen-2-yl] acetate structure](https://image.chemsrc.com/caspic/043/84993-87-3.png)