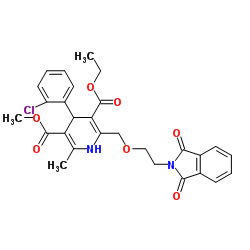
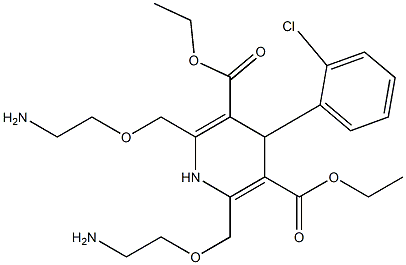
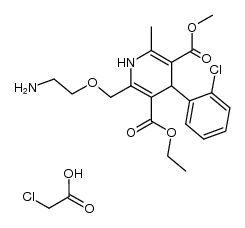
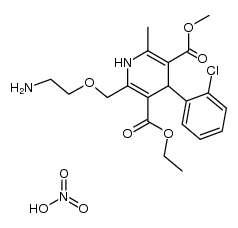
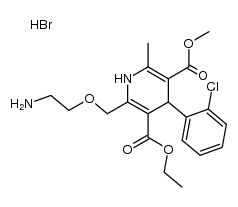
111470-99-6
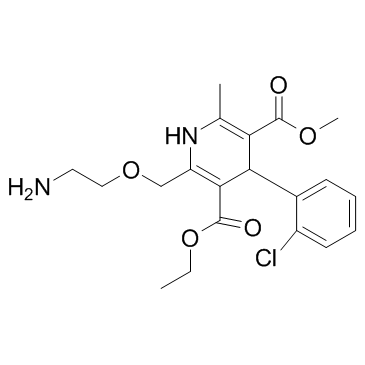
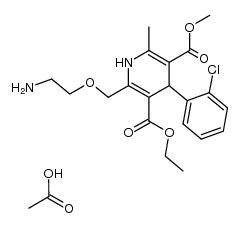
| Name | amlodipine benzenesulfonate |
|---|---|
| Synonyms |
Amlodin
Norvasc 3-Ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzenesulfonate (1:1) 3-Ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydro-3,5-pyridinedicarboxylate benzenesulfonate (1:1) 3,5-Pyridinedicarboxylic acid, 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-, 3-ethyl 5-methyl ester, benzenesulfonate (1:1) Benzolsulfonsäure--3-ethyl-5-methyl-2-[(2-aminoethoxy)methyl]-4-(2-chlorphenyl)-6-methyl-1,4-dihydropyridin-3,5-dicarboxylat(1:1) UNII:864V2Q084H MFCD00887594 acide benzènesulfonique - 2-[(2-aminoéthoxy)méthyl]-4-(2-chlorophényl)-6-méthyl-1,4-dihydropyridine-3,5-dicarboxylate de 3-éthyle et de 5-méthyle (1:1) 3-ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate benzenesulfonate 3-Ethyl 5-methyl 2-[(2-aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydro-3,5-pyridinedicarboxylate benzenesulfonate Amlodipine Besilate Amlodipine besylate benzenesulfonic acid,3-O-ethyl 5-O-methyl 2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-6-methyl-1,4-dihydropyridine-3,5-dicarboxylate AMLODIPINE BENZENESULFONATE 3-Ethyl 5-Methyl (±)-2-[(2-Aminoethoxy)methyl]-4-(2-chlorophenyl)-6-methyl-1,4-dihydro-3,5-pyridinedicarboxylate Monobenzenesulfonate |
| Description | Amlodipine besylate is a long-acting calcium channel blocker.Target: Calcium ChannelAmlodipine is a dihydropyridine calcium antagonist (calcium ion antagonist or slow-channel blocker) that inhibits the movement of calcium ions into vascular smooth muscle cells and cardiac muscle cells. Experimental data suggest amlodipine binds to both dihydropyridine and nondihydropyridine binding sites. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. Amlodipine inhibits calcium ion influx across cell membranes selectively, with a greater effect on vascular smooth muscle cells than on cardiac muscle cells. Negative inotropic effects, or decreased heart muscle contractility, can be detected in vitro, but such effects have not been seen in intact animals at therapeutic doses. Serum calcium concentration is not affected by amlodipine. Within the physiologic pH range, amlodipine is an ionized compound (pKa = 8.6), and its interaction with the calcium channel receptor is characterized by a gradual rate of association and dissociation with the receptor binding site, resulting in a gradual onset of effect. Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and reduction in blood pressure. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.227g/cm3 |
|---|---|
| Boiling Point | 527.2ºC at 760 mmHg |
| Melting Point | 199-201°C |
| Molecular Formula | C26H31ClN2O8S |
| Molecular Weight | 567.051 |
| Flash Point | 272.6ºC |
| Exact Mass | 566.148987 |
| PSA | 162.63000 |
| LogP | 5.30950 |
| Vapour Pressure | 3.34E-11mmHg at 25°C |
| Storage condition | Store at RT |
| Water Solubility | DMSO: 20 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | US7967700 |
| HS Code | 2933990090 |
|
~87% 
111470-99-6 |
| Literature: EOS ECZACIBASI OZGUN KIMYASAL URUNLER SANAYI VE TICARET A.S. Patent: WO2004/58711 A1, 2004 ; Location in patent: Page 16, 17 ; |
|
~88% 
111470-99-6 |
| Literature: Adamed SP. Z O.O. Patent: EP993447 B1, 2005 ; Location in patent: Page/Page column 3 ; |
|
~81% 
111470-99-6 |
| Literature: Adamed SP. Z O.O. Patent: EP993447 B1, 2005 ; Location in patent: Page/Page column 3 ; |
|
~% 
111470-99-6 |
| Literature: WO2011/117876 A1, ; Page/Page column 11-12 ; |
|
~%
Detail
|
| Literature: WO2003/101965 A1, ; Page 18; 19; 23-25 ; |
|
~88% 
111470-99-6 |
| Literature: Adamed SP. Z O.O. Patent: EP993447 B1, 2005 ; Location in patent: Page/Page column 3 ; |
|
~81% 
111470-99-6 |
| Literature: Adamed SP. Z O.O. Patent: EP993447 B1, 2005 ; Location in patent: Page/Page column 4 ; |
|
~% 
111470-99-6 |
| Literature: WO2007/131759 A1, ; Page/Page column 8-10 ; |
|
~% 
111470-99-6 |
| Literature: EP993447 B1, ; Page/Page column 3 ; |
| Precursor 9 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |