4382-63-2
| Name | pfk15 |
|---|---|
| Synonyms |
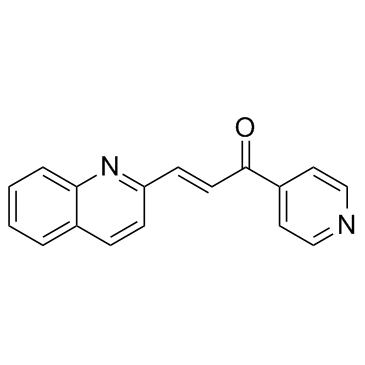
(2E)-1-(4-Pyridinyl)-3-(2-quinolinyl)-2-propen-1-one
1-(4-Pyridinyl)-3-(2-quinolinyl)-2-propen-1-one PFK-015 |
| Description | PFK-015 is an effective inhibitor of PFKFB3 with IC50 of 110 nM (recombinant PFKFB3) and inhibits PFKFB3 activity in cancer cells with IC50 of 20 nM.IC50 value: 110 nM (recombinant PFKFB3)[1]Target: PFKFB3 PFK-015 possesses compelling in vitro properties, has satisfactory PK properties in rodents, and suppresses tumor glucose metabolism and growth in an aggressive mouse model of non-small cell lung cancer. PFK-015 is not a Pgp substrate as determined by transport and cell permeability assays in Caco-2 and MDCK-MDR1 (Papp A-B / B-A results 1.8 / 4 and 5 / 5 10–6 cm/s). PFK-015 inhibits cancer cell proliferation in a panel of 17 cancer cell lines. PFK-015 suppresses glucose uptake in cancer cells. Rodent PK studies following IV dosing at 5 mg/kg resulted in a profile with a satisfactory half-life (5.1 hours), exposure (AUCinf 1804 ng.h/ml), tissue distribution (Vd 20.5 L/kg) and reasonable clearance (46.2 mL/min/kg). Also, pre-clinical efficacy studies of C57Bl/6 mice bearing Lewis Lung Carcinoma (LLC) xenografts demonstrated 80% tumor growth inhibition relative to vehicle control. Finally, micro-PET studies performed on mice bearing LLC tumors showed a significant inhibition of tumor 2-[18F]-fluoro-2-deoxy-glucose uptake. These results support further development of PFK-015 as a novel anti-cancer agent. |
|---|---|
| Related Catalog | |
| References |
[1]. Pfkfb3 inhibitor and methods of use as an anti-cancer therapeutic |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 464.4±45.0 °C at 760 mmHg |
| Molecular Formula | C17H12N2O |
| Molecular Weight | 260.290 |
| Flash Point | 233.5±35.1 °C |
| Exact Mass | 260.094971 |
| PSA | 42.85000 |
| LogP | 2.62 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.698 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |