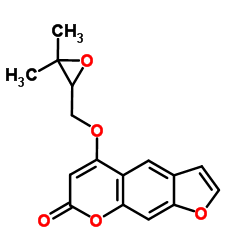
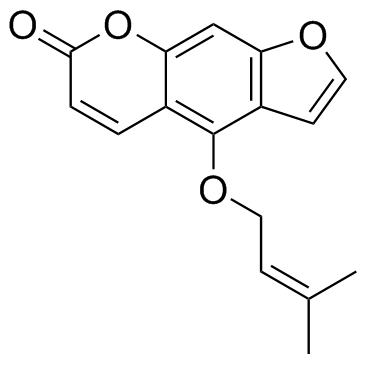
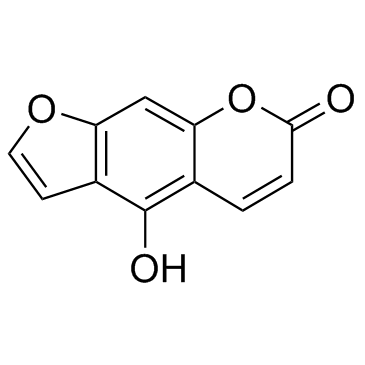
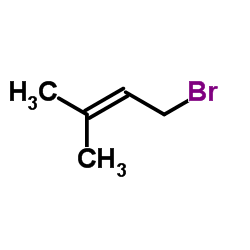
2643-85-8
| Name | Oxypeucedanin hydrate |
|---|---|
| Synonyms |
4-[(2R)-2,3-Dihydroxy-3-methylbutoxy]-7H-furo[3,2-g]chromen-7-one
oxypeucedaninhydrate |
| Description | Oxypeucedanin hydrate ((+)-Oxypeucedanin hydrate) is a natural product isolated from D. anethifolia. Prangol exhibits mild toxicity on fibroblasts and parental lymphoma cells[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 544.3±50.0 °C at 760 mmHg |
| Molecular Formula | C16H16O6 |
| Molecular Weight | 304.295 |
| Flash Point | 283.0±30.1 °C |
| Exact Mass | 304.094696 |
| PSA | 93.04000 |
| LogP | 1.31 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.630 |
| Storage condition | 2-8℃ |
|
~92% 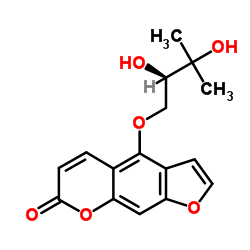
2643-85-8 |
| Literature: Row; Brown; Stachulski; Lennard Organic and Biomolecular Chemistry, 2006 , vol. 4, # 8 p. 1604 - 1610 |
|
~% 
2643-85-8 |
| Literature: Row; Brown; Stachulski; Lennard Organic and Biomolecular Chemistry, 2006 , vol. 4, # 8 p. 1604 - 1610 |
|
~% 
2643-85-8 |
| Literature: Row; Brown; Stachulski; Lennard Organic and Biomolecular Chemistry, 2006 , vol. 4, # 8 p. 1604 - 1610 |
|
~% 
2643-85-8 |
| Literature: Row; Brown; Stachulski; Lennard Organic and Biomolecular Chemistry, 2006 , vol. 4, # 8 p. 1604 - 1610 |
|
~% 
2643-85-8 |
| Literature: Row; Brown; Stachulski; Lennard Organic and Biomolecular Chemistry, 2006 , vol. 4, # 8 p. 1604 - 1610 |