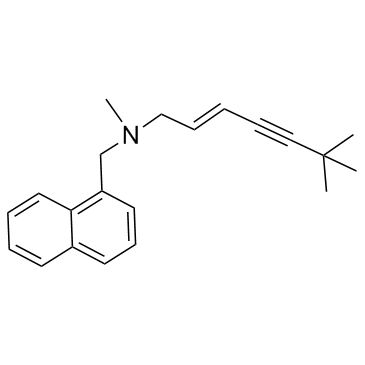
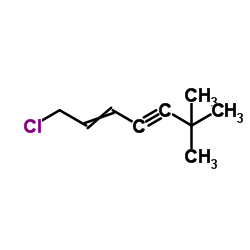
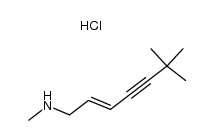
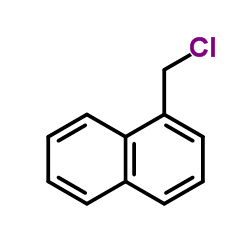
78628-80-5
| Name | terbinafine hydrochloride |
|---|---|
| Synonyms |
(2E)-N,6,6-Trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-in-1-aminhydrochlorid
(2E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine hydrochloride (1:1) 1-Naphthalenemethanamine, N-[(2E)-6,6-dimethyl-2-hepten-4-yn-1-yl]-N-methyl-, hydrochloride (1:1) MFCD00145430 (2E)-N,6,6-Trimethyl-N-(1-naphthylmethyl)-2-hepten-4-yn-1-amine hydrochloride (1:1) (2E)-N,6,6-Trimethyl-N-(1-naphthylmethyl)hept-2-en-4-yn-1-amine hydrochloride (1:1) Lamisil trans-N-Methyl-N-(1-naphthylmethyl)-6,6-dimethylhept-2-en-4-ynyl-1-amine hydrochloride (2E)-N,6,6-trimethyl-N-(naphthalen-1-ylmethyl)hept-2-en-4-yn-1-amine hydrochloride Terbinafine HCl Terbinafine hydrochloride EINECS 245-385-8 (E)-N-(6,6-Dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine monohydrochloride |
| Description | Terbinafine hydrochloride (TDT 067 hydrochloride) is an antifungal medication used to treat fungal infections. It is a potent non-competitive inhibitor of squalene epoxidase from Candida with a Ki of 30 nM. |
|---|---|
| Related Catalog | |
| Target |
Ki: 30 nM (squalene epoxidase)[1] |
| In Vitro | Terbinafine has a primary fungicidal action in vitro against most fungal pathogens, including dermatophytes, and dimorphic and filamentous fungi. Terbinafine specifically inhibits fungal ergosterol biosynthesis at the point of squalene epoxidation. The treated fungal cells rapidly accumulate tlic intermediate squalene and become deficient in the end-product of the pathway, ergosterol[1]. |
| In Vivo | Terbinafine is not only active after topical application but is very effective in experimental dermatophytoses following oral administration. In fungi infected guinea-pigs, the skin temperature dropps dramatically after the fourth treatment of terbinafine[2]. |
| Animal Admin | Guinea-pigs: The backs (lumbar regions) of guinea-pigs, which have been mechanically depilated, are infected with 0.1 mL Sabouraud's dextrose 2% broth containing 106 c.f.u. of Truhophyton mentagrophytes. The treatments commence 48 h post-inoculation. The test compounds (Terbinafine) are suspended in 2% tylose and Tween 80 and administered via a stomach tube once daily on 9 consecutive days, or dissolved in a mixture of polyethylene glycol 400 and etbanol and spread on the infected part ot the body in a volume of 0.4 mL with a Hrigalski spatula once daily for 1-7 consecutive days[2]. |
| References |
| Density | 1.007g/cm3 |
|---|---|
| Boiling Point | 417.9ºC at 760 mmHg |
| Melting Point | 204-208°C |
| Molecular Formula | C21H26ClN |
| Molecular Weight | 327.891 |
| Flash Point | 183.7ºC |
| Exact Mass | 327.175385 |
| PSA | 3.24000 |
| LogP | 5.67930 |
| Index of Refraction | 1.586 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335-H410 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant;N: Dangerous for the environment; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39-S61-S60 |
| RIDADR | UN 3077 9/PG 3 |
| RTECS | QJ8600100 |
| HS Code | 2921499090 |
|
~80% 
78628-80-5 |
| Literature: HYDEX CHEMICALS PVT LTD. Patent: WO2005/110968 A1, 2005 ; Location in patent: Page/Page column 9-10 ; |
|
~77% 
78628-80-5 |
| Literature: F.I.S. FABBRICA ITALIANA SINTETICI S.P.A.; DELLA NEGRA, Frederico Patent: WO2005/121155 A1, 2005 ; Location in patent: Page/Page column 37-38 ; |
|
~% 
78628-80-5 |
| Literature: WO2007/52089 A1, ; Page/Page column 7 ; |
|
~% 
78628-80-5 |
| Literature: US2006/84826 A1, ; Page/Page column 3 ; |
|
~% 
78628-80-5 |
| Literature: US5231183 A1, ; US 5231183 A |
|
~% 
78628-80-5 |
| Literature: WO2007/44273 A1, ; Page/Page column 16; 17 ; |
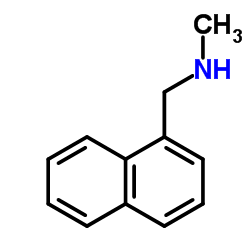
| Precursor 8 | |
|---|---|
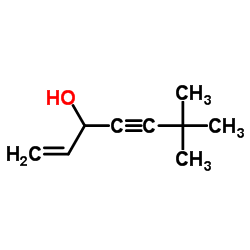
| DownStream 1 | |
| HS Code | 2921499090 |
|---|---|
| Summary | 2921499090 other aromatic monoamines and their derivatives; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |