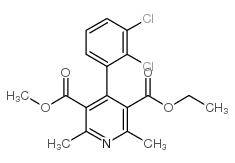
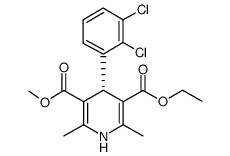
72509-76-3
| Name | felodipine |
|---|---|
| Synonyms |
4-(2,3-Dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic acid ethyl methyl ester
4-(2,3-Dichloro-phenyl)-2,6-dimethyl-1,4-dihydro-pyridine-3,5-dicarboxylic acid 3-ethyl ester 5-methyl ester Splendil Flodil Feloday Munobal T6M DHJ B1 CVO2 DR BG CG& EVO1 F1 MFCD08235033 3,5-Pyridinedicarboxylic acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, ethyl methyl ester (RS)-Felodipine Ethyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydro-3,5-pyridinedicarboxylate Hydac modip P|endil Felodipine Plendil Ethyl-methyl-4-(2,3-dichlorphenyl)-2,6-dimethyl-1,4-dihydropyridin-3,5-dicarboxylat CGH-869 Spendil Renedil h154/82 (±)-Felodipine Ethyl methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate Agon Prevex |
| Description | Felodipine is a long-acting 1,4-dihydropyridine calcium channel blocker.Target: Calcium ChannelFelodipine is a long-acting 1,4-dihydropyridine calcium channel blocker (CCB)b. It acts primarily on vascular smooth muscle cells by stabilizing voltage-gated L-type calcium channels in their inactive conformation. Felodipine significantly relaxes KCl-contracted porcine coronary segments by blocking the Ca2+ channels, displaying ~50 times more potent than nifedipine (IC50 of ~8 nM) and ~430 times than verapamil (IC50 of ~65 nM) [1]. Felodipine significantly induces the transcription and secretion of IL-6 and IL-8 with ED50 values of 5.8 nM and 5.3 nM in primary human VSMC and lung fibroblasts, respectively, while propranolol or furosemide fails to affect the expression of the two IL genes [2]. Felodipine blocks the muscarinic receptor-mediated (carbachol) Ca2+-dependent contraction of guinea pig ileum longitudinal smooth muscle (GPILSM) with an IC50 of 1.45 nM [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 471.5±45.0 °C at 760 mmHg |
| Melting Point | 142-145°C |
| Molecular Formula | C18H19Cl2NO4 |
| Molecular Weight | 384.254 |
| Flash Point | 239.0±28.7 °C |
| Exact Mass | 383.069122 |
| PSA | 64.63000 |
| LogP | 4.83 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.550 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | US7968700 |
| HS Code | 2933990090 |
|
~84% 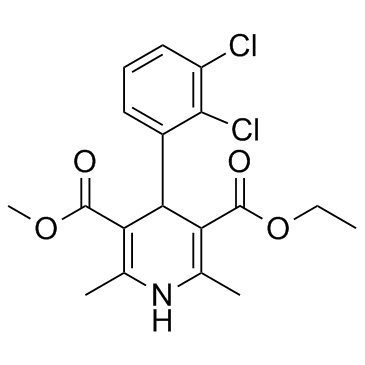
72509-76-3 |
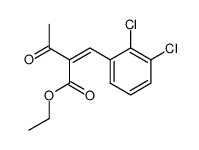
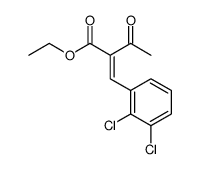
| Literature: Palacios, Francisco; Herran, Esther; Rubiales, Gloria; Alonso, Concepcion Tetrahedron, 2007 , vol. 63, # 25 p. 5669 - 5676 |
|
~97% 
72509-76-3 |
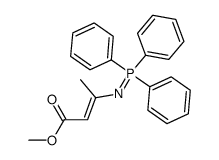
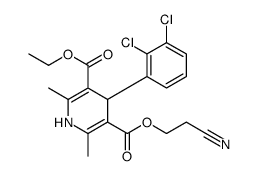
| Literature: Alajarin; Jordan; Vaquero; Alvarez-Builla Synthesis, 1995 , # 4 p. 389 - 391 |
|
~84% 
72509-76-3 |
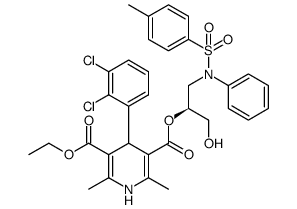
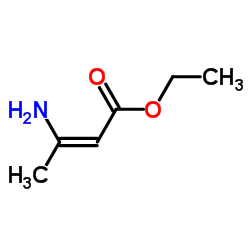
| Literature: Kwon, Kuktae; Shin, Jung A.; Lee, Hee-Yoon Tetrahedron, 2011 , vol. 67, # 52 p. 10222 - 10228 |
|
~% 
72509-76-3 |
| Literature: Organic Preparations and Procedures International, , vol. 28, # 1 p. 91 - 95 |
|
~% 
72509-76-3 |
| Literature: Organic Preparations and Procedures International, , vol. 28, # 1 p. 91 - 95 |
|
~% 
72509-76-3 |
| Literature: Tetrahedron, , vol. 67, # 52 p. 10222 - 10228 |
|
~% 
72509-76-3 |
| Literature: Tetrahedron, , vol. 67, # 52 p. 10222 - 10228 |
|
~% 
72509-76-3 |
| Literature: Tetrahedron, , vol. 67, # 52 p. 10222 - 10228 |
|
~% 
72509-76-3 |
| Literature: WO2012/123966 A1, ; Page/Page column 29 ; |
| Precursor 7 | |
|---|---|
| DownStream 3 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |