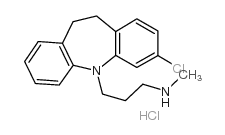
303-49-1
| Name | clomipramine |
|---|---|
| Synonyms |
3-Chloroimipramine
Chlorimipramine 3-Chloro-10,11-dihydro-N,N-dimethyl-5H-dibenz[b,f]azepine-5-propanamine MFCD00069234 Clomipramine 3-(3-chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethylpropan-1-amine 5-(g-Dimethylaminopropyl)-3-chloroiminodibenzyl 3-Chloro-10,11-dihydro-5-(3-dimethylaminopropyl)-5H-dibenz[b,f]azepine 3-chloro-10,11-dihydro-N,N-dimethyl-5H-Dibenz(b,f)azepine-5-propanamine Hydiphen Clomipramina 10,11-dihydro-3-chloro-5-(3-(dimethylamino)propyl)-5H-dibenz(b,f)azepine 3-Chloro-5-[3-(dimethylamino)propyl]-10,11-dihydro-5H-dibenz[b,f]azepine 3-(3-Chloro-10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N,N-dimethyl-1-propanamine Monochlorimipramine ANAFRANIL 3-(2-chloro-5,6-dihydrobenzo[b][1]benzazepin-11-yl)-N,N-dimethylpropan-1-amine chloripramine EINECS 206-144-2 Chlomipramine Anafranil base |
| Description | Clomipramine (Chlorimipramine) is a potent 5-HT reuptake blocker with the IC50 value of 1.5 nM. Clomipramine is a tricyclic antidepressant that can be used for the research of depression and obsessive compulsive disorder (OCD)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Clomipramine can inhibit reuptake of both noradrenaline and 5-HT, although Clomipramine inhibits 5-HT reuptake more strongly than it inhibits noradrenaline reuptake[1]. The antidepressant Clomipramine inhibits both venom AChE as well as human serum BChE in a concentration-dependent manner but has no effect on AChE in the rat brain striatum[2]. Clomipramine interferes with the autophagic flux and severely compromises the viability of tumorigenic cells upon cytotoxic stress[3]. Clomipramine reduces autophagy in neuronal primary cultures. Clomipramine (1 and 5 µM) negatively regulates neuronal autophagic pathway in primary cultured cells[3]. Western Blot Analysis[3] Cell Line: Primary cortical neurons Concentration: 1 and 5 µM Incubation Time: 12, 24 and 48 hours Result: Enhanced the LC3-I conversion to LC3-II in a concentration-dependent manner at all analyzed time points. |
| In Vivo | Clomipramine (5-20 mg/kg; i.p) elicits significant hyperglycemia in mice. Clomipramine induces hyperglycemia in mice by blocking the 5-HT2B and/or 5-HT2C receptors, which results in facilitation of adrenaline release. In mice, Clomipramine reduces immobility in the forced swimming test, which is the behavioral model for antidepressants. Clomipramine also inhibits the OCD animal model, marble burying behavior in mice[1]. Clomipramine (20 mg/kg) decreases autophagic flux in murine tissues[3]. Animal Model: C57BL/6 J mice (6 weeks of age and 22 to 25 g)[3] Dosage: 20 mg/kg Administration: Treated intraperitoneally for 21 days Result: Both LC3-II and p62 were significantly increased in the liver of Clomipramine treated mice compared to vehicle treated ones. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 434.2±45.0 °C at 760 mmHg |
| Molecular Formula | C19H23ClN2 |
| Molecular Weight | 314.852 |
| Flash Point | 216.4±28.7 °C |
| Exact Mass | 314.154968 |
| PSA | 6.48000 |
| LogP | 5.39 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Water Solubility | H2O: 25 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36 |
| WGK Germany | 3 |
| RTECS | HN9055000 |
| HS Code | 2933990090 |
|
~83% 
303-49-1 |
| Literature: Tetrahedron Letters, , vol. 51, # 43 p. 5690 - 5693 |
| Precursor 1 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |