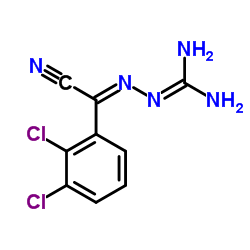
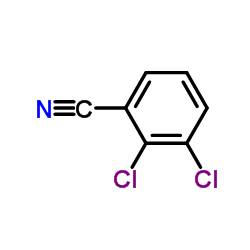
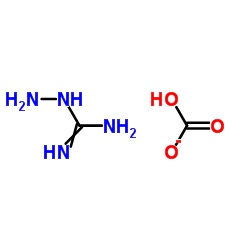
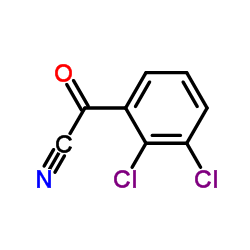
84057-84-1
| Name | lamotrigine |
|---|---|
| Synonyms |
Lamictal
Lamotrigin 6-(2,3-Dichlorophenyl)-1,2,4-triazine-3,5-diamine EINECS 281-901-8 6-(2,3-Dichlorophenyl)-3-imino-3,4-dihydro-1,2,4-triazin-5-amine Crisomet Lamictal CD HYDROXYMETHYL PROGESTERONE 6-O-TRITYL-1,2,3,4-TETRA-O-ACETYL-ALPHA-D-MANNOPYRANOSE BW 430C LAMOTRIGENE LAMOTRIGINE API MFCD00865333 LAMOTRIGINE / 6-(2,3-DICHLOROPHENYL)-1,2,4-TRIAZINE-3,5-DIAMINE Lamictal XR LAMOTRIGINE-13C3 LAMOTRIGINE (10 MG ) Lamotrigina Labileno Lamotriginum Lamotrigine |
| Description | Lamotrigine(BW430C) is a novel anticonvulsant drug for inhibition of 5-HT and sodium channelTarget: Sodium ChannelLamotrigine stabilises presynaptic neuronal membranes by blockade of voltage-dependent sodium channels, thus preventing the release of excitatory neurotransmitters, particularly glutamate and aspartate [1]. In rat cerebral cortex tissue incubated with veratrine 10 mg/L, lamotrigine is twice as potent in inhibiting the release of glutamate and aspartate (ED 50 = 5.38 mg/L for each) than the release of GABA (ED50 = 11.2 mg/L), and is much less potent in inhibiting acetylcholine release (ED50 = 25.6 mg/L) when cortical slices is exposed to veratrine 75 mg/L. Basal glutamate release is unaffected [2]. Lamotrigine inhibits high-frequency sustained repetitive firing of sodium-dependent action potentials, indicating a direct effect on voltage-activated sodium channels [3]. Lamotrigine (Lamictal), a phenyltriazine derivative, is a well established anticonvulsant agent that has shown efficacy in the prevention of mood episodes in adult patients with bipolar I disorder. lamotrigine significantly delayed time to intervention for a depressive episode and showed limited efficacy in delaying time to intervention for a manic/hypomanic episode, compared with placebo. Lamotrigine is generally well tolerated [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 503.1±60.0 °C at 760 mmHg |
| Melting Point | 177-181°C |
| Molecular Formula | C9H7Cl2N5 |
| Molecular Weight | 256.091 |
| Flash Point | 258.1±32.9 °C |
| Exact Mass | 255.007858 |
| PSA | 90.71000 |
| LogP | -0.19 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.706 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: 20 mg/mL at 60 °C, soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | XY5850700 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933699090 |
|
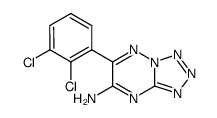
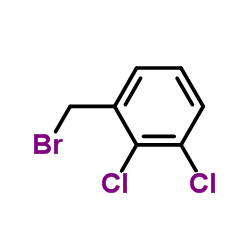
~82% 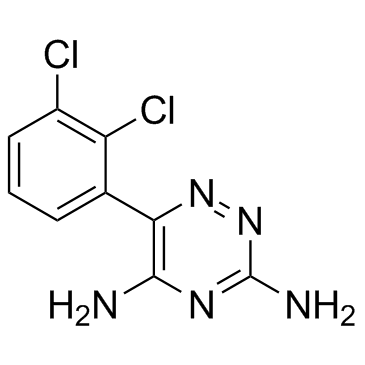
84057-84-1 |
| Literature: ALEMBIC LIMITED Patent: WO2009/69073 A1, 2009 ; Location in patent: Page/Page column 3; 5 ; |
|
~% 
84057-84-1 |
| Literature: US4847249 A1, ; |
|
~% 
84057-84-1 |
| Literature: US6111101 A1, ; |
|
~% 
84057-84-1 |
| Literature: EP247892 B1, ; |
|
~76% 
84057-84-1 |
| Literature: Ulomskii; Shestakova; Deev; Rusinov; Chupakhin Russian Chemical Bulletin, 2005 , vol. 54, # 3 p. 726 - 732 |
|
~% 
84057-84-1 |
| Literature: Russian Chemical Bulletin, , vol. 54, # 3 p. 726 - 732 |
|
~% 
84057-84-1 |
| Literature: Russian Chemical Bulletin, , vol. 54, # 3 p. 726 - 732 |
|
~% 
84057-84-1 |
| Literature: Russian Chemical Bulletin, , vol. 54, # 3 p. 726 - 732 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |