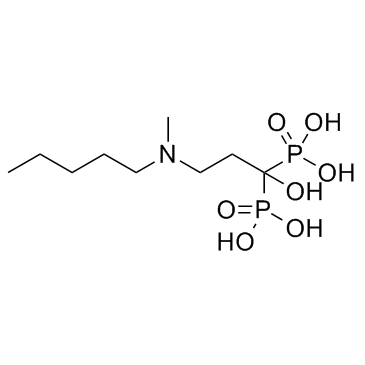
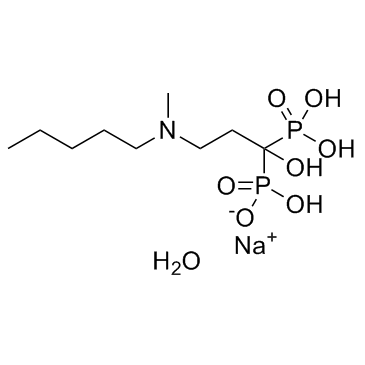
138926-19-9
| Name | Ibandronate Sodium Monohydrate |
|---|---|
| Synonyms |
BM-21.0955 monosodium salt monohydrate
Sodium trihydrogen (1-hydroxy-3-(methylpentylamino)propylidene)diphosphonate, monohydrate. Phosphonic acid, [1-hydroxy-3-(methylpentylamino)propylidene]bis-, sodium salt, hydrate (1:1:1) Ibandronic acid monosodium salt monohydrate Ibandronate sodium hydrate Ibandronate sodium monohydrate sodium,hydroxy-[1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl]phosphinate,hydrate BM 21.0955Na.H2O (1-Hydroxy-3-(methylpentylamino)propylidene)bisphosphonic acid sodium Sodium hydrogen {1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl}phosphonate hydrate (1:1:1) Phosphonic acid, (1-hydroxy-3-(methylpentylamino)propylidene)bis-, monosodium salt, monohydrate MFCD00912167 Ibandronate (Sodium Monohydrate) |
| Description | Ibandronate is a highly potent nitrogen-containing bisphosphonate used for the treatment of osteoporosis.Target: OthersIbandronate (1.25-2 μM) significantly reduces endothelial cell growth, while ibandronate (2 μM) also significantly reduces capillary-like tube formation and increases apoptosis of endothelial cells. Ibandronate (< 100 μM) dose-dependently increases VEGF expression in endothelial cells [1]. Ibandronate (< 100 μM) inhibits growth of both prostate cancer cell lines (LNCaP and PC-3) in a dose dependent manner [2].Ibandronate administered either daily (2.5 mg) or intermittently (20 mg every other day for 12 doses every 3 months) significantly reduces the risk of new morphometric vertebral fractures by 62% and 50% (p = 0.0006), respectively, in osteoporotic women after 3 years' treatment. Ibandronate administered either daily (2.5 mg) or intermittently (20 mg every other day for 12 doses every 3 months) significantly and progressively increases BMD of lumbar spine by 6.5% and 5.7%, respectively, in osteoporotic women after 3 years' treatment [3]. Ibandronate (< 125 mg/kg s.c.) results in a dose dependent increase in bone mineral density (BMD), trabecular bone volume and trabecular number, load to failure (Fmax), and yield load in long bones and vertebrae in ovariectomized rats, and increased trabecular separation in ovariectomized rats is fully prevented by all doses [4]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 587.8ºC at 760 mmHg |
|---|---|
| Melting Point | 84ºC (dec) |
| Molecular Formula | C9H24NNaO8P2 |
| Molecular Weight | 359.226 |
| Flash Point | 309.3ºC |
| Exact Mass | 359.087494 |
| PSA | 170.21000 |
| LogP | 0.87390 |
| Vapour Pressure | 2.88E-16mmHg at 25°C |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R40 |
| Safety Phrases | 22-36 |
| RIDADR | UN 1759 8 / PGIII |
| HS Code | 2922199090 |
|
~% 
138926-19-9 |
| Literature: WO2005/63779 A2, ; Page/Page column 6 ; |
|
~% 
138926-19-9 |
| Literature: WO2011/16738 A1, ; Page/Page column 9-10 ; |
|
~% 
138926-19-9 |
| Literature: WO2008/60609 A1, ; Page/Page column 21 ; |
|
~% 
138926-19-9 |
| Literature: WO2008/14510 A2, ; Page/Page column 24-25 ; |
|
~% 
138926-19-9 |
| Literature: WO2008/60609 A1, ; Page/Page column 21 ; |
|
~% 
138926-19-9 |
| Literature: WO2008/60609 A1, ; Page/Page column 22 ; |
| Precursor 4 | |
|---|---|
| DownStream 0 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |