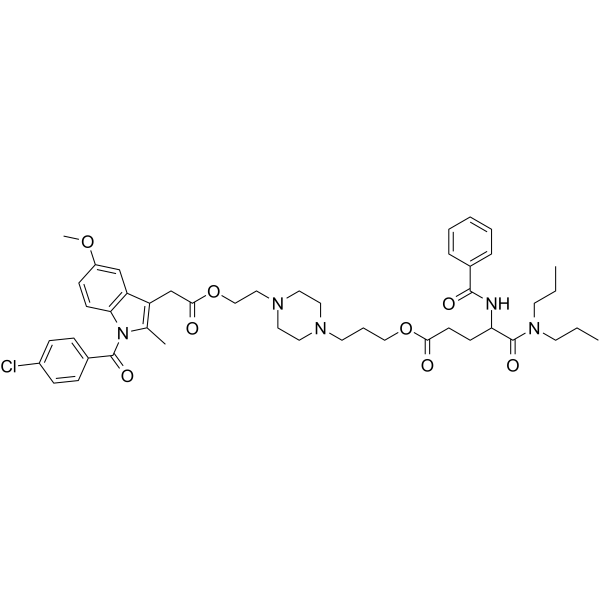
57132-53-3
| Name | proglumetacin |
|---|---|
| Synonyms |
PROGLUMETACIN
Proglumetacina Proglumetacine 3-[4-[2-[2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetyl]oxyethyl]piperazin-1-yl]propyl 4-benzamido-5-(dipropylamino)-5-oxopentanoate Proglumetacinum |
| Description | Proglumetacin is an orally active and potent cyclo-oxygenase inhibitor. Proglumetacin can inhibits SARS-CoV Mpro (main protease of the SARS-CoV-2), with an AC50 of 8.9 μM (activity concentration at half maximal activity). Proglumetacin has anti-inflammatory activity, can be used for inflammation (such as Rheumatoid arthritis, and Allergic air pouch inflammation) research[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Proglumetacin strongly inhibits 5-HETE formation, with an IC50 of 1.5 μM[2]. Proglumetacin inhibits leukocyte migration by inhibiting the production of the chemotactic cyclo-oxygenase product thromboxane B2[2]. |
| In Vivo | Proglumetacin (Sprague-Dawley rats, 0-30 mg/kg, Orally, once) dose-dependently inhibits accumulation of pouch exudate[1]. Animal Model: Sprague-Dawley rats (6 weeks) Dosage: 0, 0.3, 3, 9, 30 mg/kg Administration: Orally, once Result: Caused dose-dependent reduction of leukocyte migration into the pouch exudate, caused 49.2% inhibition at 30 mg/kg; and markedly decreased the prostaglandin E2 content of the pouch exudate, but tended to increase the leukotriene B 4 content. |
| References |
| Density | 1.22 g/cm3 |
|---|---|
| Boiling Point | 900.4ºC at 760 mmHg |
| Molecular Formula | C46H58ClN5O8 |
| Molecular Weight | 844.43 |
| Flash Point | 498.3ºC |
| Exact Mass | 843.39700 |
| PSA | 139.72000 |
| LogP | 6.43090 |
| Index of Refraction | 1.592 |