52809-07-1
| Name | (+)-Quisqualic Acid |
|---|---|
| Synonyms |
L-Quisqualic acid
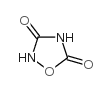
MFCD00069337 3-(3,5-Dioxo-1,2,4-oxadiazolidin-2-yl)-L-alanine b-(3,5-Dioxo-1,2,4-oxodiazolidin-2-yl)-L-alanine L-(+)-a-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic Acid (L)-(+)-a-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic acid Quisqualic acid 3-(3-Hydroxy-5-oxo-1,2,4-oxadiazol-2(5H)-yl)-L-alanine β-(3,5-Dioxo-1,2,4-oxadiazolidin-2-yl)-L-alanine L-Quisqualic acid,(L)-(+)-α-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoicacid (S)-a-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic Acid |
| Description | Quisqualic acid (L-Quisqualic acid), a natural analog of glutamate, is a potent and pan two subsets (iGluR and mGluR) of excitatory amino acid (EAA) agonist with an EC50 of 45 nM and a Ki of 10 nM for mGluR1R. Quisqualic acid is isolated from the fruits of Quisqualis chinensis[1][2]. |
|---|---|
| Related Catalog | |
| Target |
mGluR1R:45 nM (EC50) mGluR1R:10 nM (Ki) mGluR2R:108 μM (IC50) mGluR2R:113 μM (Ki) mGluR4R:593 μM (IC50) mGluR4R:112 μM (Ki) |
| In Vitro | Quisqualic acid is an agonist of AMPA and metabotropic glutamate receptors. Quisqualic acid activates mGluR2R (EC50=108 μM; Ki=113 μM) and mGluR4R (EC50=593 μM; Ki=112 μM)[1]. |
| References |
| Density | 2.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 405.9±55.0 °C at 760 mmHg |
| Melting Point | 185-187ºC dec. |
| Molecular Formula | C5H7N3O5 |
| Molecular Weight | 189.126 |
| Flash Point | 199.3±31.5 °C |
| Exact Mass | 189.038574 |
| PSA | 131.32000 |
| LogP | -1.85 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.726 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R20/21/22 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
|
~10% 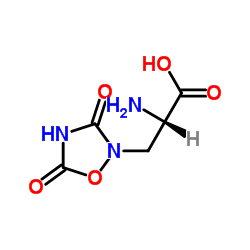
52809-07-1 |
| Literature: Murakoshi, Isamu; Ikegami, Fumio; Yoneda, Yoshihiro; Ihara, Harumi; Sakata, Kumiko; Koide, Chiharu Chemical & Pharmaceutical Bulletin, 1986 , vol. 34, # 4 p. 1473 - 1478 |
|
~% 
52809-07-1 |
| Literature: Ikegami, Fumio; Takayama, Kyoko; Tajima, Chiho; Murakoshi, Isamu Phytochemistry (Elsevier), 1988 , vol. 27, # 7 p. 2011 - 2016 |
|
~% 
52809-07-1 |
| Literature: Baldwin, Jack E.; Adlington, Robert M.; Birch, David J. Journal of the Chemical Society, Chemical Communications, 1985 , # 5 p. 256 - 257 |
|
~% 
52809-07-1 |
| Literature: Murakoshi, Isamu; Ikegami, Fumio; Koide, Chiharu Heterocycles, 1984 , vol. 21, # 2 p. 705 |
| Precursor 4 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |