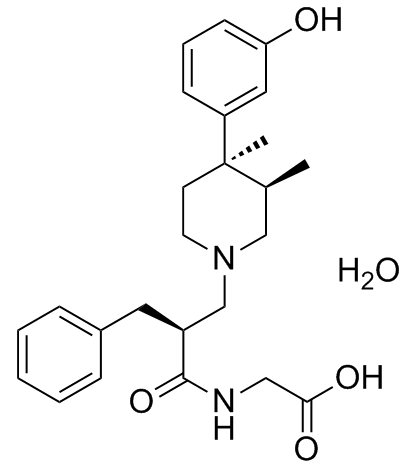
1383577-62-5
| Name | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic acid,hydrate |
|---|---|
| Synonyms |
((2S)-2-(((3R,4R)-4-(3-Hydroxyphenyl)-3,4-dimethylpiperidin-1-yl)methyl)-3-phenylpropanoyl)amino)acetic acid monohydrate
Alvimopan monohydrate 28LAR2REDG UNII-28LAR2REDG Alvimopan (monohydrate) |
| Description | Alvimopan monohydrate is a peripherally acting mu-opioid receptor (PAM-OR, IC50= 1.7 nM) antagonist for accelerating gastrointestinal recovery after surgery. IC50 Value: 1.7 nM (Mu-type opioid receptor) [1]in vitro: The dissociation rate of alvimopan from the micro opioid receptor (t(1/2)=30--44 min) was comparable to that of the long acting partial agonist buprenorphine (t(1/2)=44 min), but was slower than those of the antagonists naloxone (t(1/2)=0.82 min) and N-methylnaltrexone (t(1/2)=0.46 min) [2].in vivo: Alvimopan did not significantly accelerate GI-3 compared with placebo [6 mg: hazard ratio (HR) = 1.20, p = 0.080; 12 mg: HR = 1.24, p = 0.038). However, after adjustment for significant covariates (sex/surgical duration), benefits were significant for both doses (6 mg: HR = 1.24, p = 0.037; 12 mg: HR = 1.26, p = 0.028). Alvimopan also significantly accelerated time to GI-2 (6 mg: HR = 1.37, p = 0.008; 12 mg: HR = 1.33, p = 0.018) and DCO (6 mg: HR = 1.31, p = 0.008; 12 mg: HR = 1.28, p = 0.015) [3]. Alvimopan (1 and 3 mg/kg) significantly reversed this delayed GI transit when administered 45 min prior to surgery. However, the effects of alvimopan were less pronounced when administered following surgery [4].Toxicity:The most common treatment-emergent adverse events across all treatment groups were nausea, vomiting, and hypotension; the incidence of nausea and vomiting was reduced by 53 percent in thealvimopan 12-mg group [5].Clinical trial: Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy. Phase 3 |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C25H34N2O5 |
|---|---|
| Molecular Weight | 442.54800 |
| Exact Mass | 442.24700 |
| PSA | 102.59000 |
| LogP | 3.76530 |