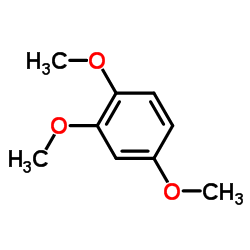
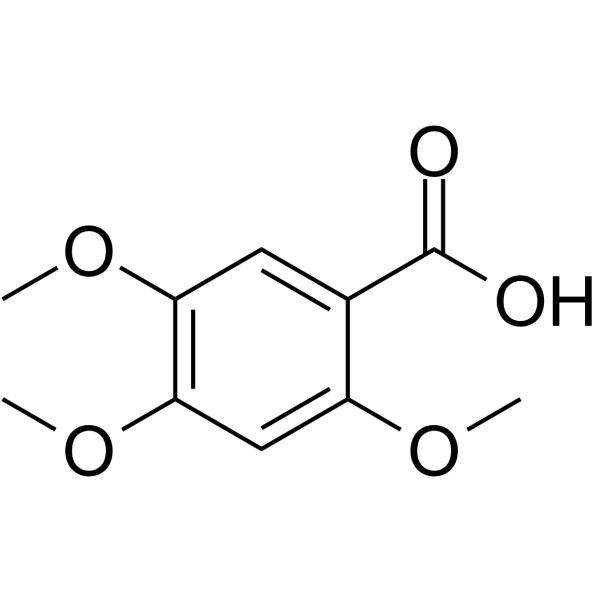
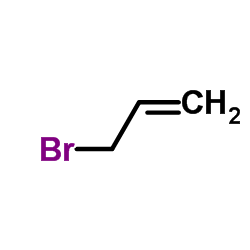
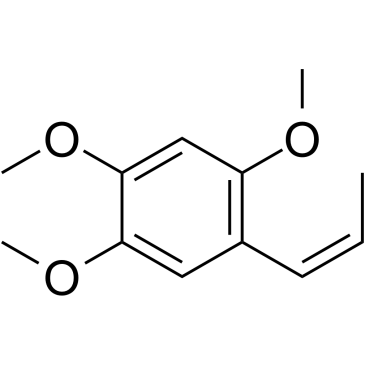
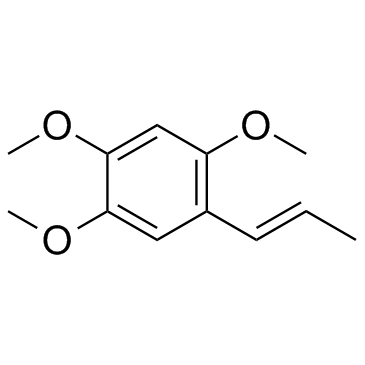
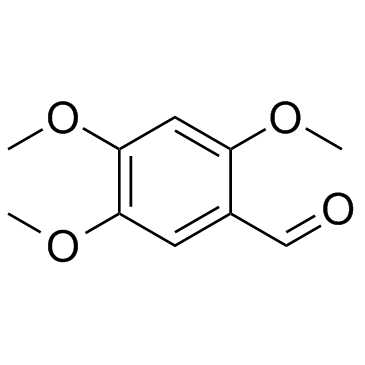
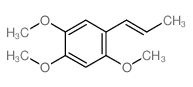
5353-15-1
| Name | 1,2,4-trimethoxy-5-prop-2-enylbenzene |
|---|---|
| Synonyms |
(2,4,5-trimethoxyphenyl)prop-1-ene
1,2,4-TRIMETHOXY-5-ALLYLBENZENE Benzene,1,2,4-trimethoxy-5-(2-propenyl) 2,4,5-Trimethoxyallylbenzene 1-Allyl-2,4,5-trimethoxy-benzol 1-allyl-2,4,5-trimethoxybenzene |
| Description | γ-Asarone, a phenylpropene, shows strong correlation with the biological activities (anti-oxidative, anti-inflammatory and neurotrophic effects)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | γ-asarone epoxide is a terminal epoxide. γ-asarone epoxide was not mutagenic under any conditions[1]. |
| References |
| Density | 1.011g/cm3 |
|---|---|
| Boiling Point | 287.7ºC at 760 mmHg |
| Molecular Formula | C12H16O3 |
| Molecular Weight | 208.25400 |
| Flash Point | 95.4ºC |
| Exact Mass | 208.11000 |
| PSA | 27.69000 |
| LogP | 2.44090 |
| Index of Refraction | 1.496 |
| HS Code | 2909309090 |
|---|
|
~89% 
5353-15-1 |
| Literature: Dieter, Janice W.; Li, Zhong; Nicholas, Kenneth M. Tetrahedron Letters, 1987 , vol. 28, # 45 p. 5415 - 5418 |
|
~44% 
5353-15-1 |
| Literature: Sinha, Arun K.; Acharya, Ruchi; Joshi, Bhupendra P. Journal of Natural Products, 2002 , vol. 65, # 5 p. 764 - 765 |
|
~87% 
5353-15-1 |
| Literature: Wang, Jiantao; Cui, Zili; Zhang, Yuexia; Li, Huajie; Wu, Long-Min; Liu, Zhongquan Organic and Biomolecular Chemistry, 2011 , vol. 9, # 3 p. 663 - 666 |
|
~% 
5353-15-1 |
| Literature: Wang, Shaopeng; Swenton, John S. Tetrahedron Letters, 1990 , vol. 31, # 11 p. 1513 - 1516 |
|
~% 
5353-15-1 |
| Literature: Sinha, Arun K.; Acharya, Ruchi; Joshi, Bhupendra P. Journal of Natural Products, 2002 , vol. 65, # 5 p. 764 - 765 |
|
~% 
5353-15-1 |
| Literature: Sinha, Arun K.; Acharya, Ruchi; Joshi, Bhupendra P. Journal of Natural Products, 2002 , vol. 65, # 5 p. 764 - 765 |
|
~% 
5353-15-1
Detail
|
| Literature: Greca, Marina Della; Monaco, Pietro; Pollio, Antonino; Previtera, Lucio Phytochemistry (Elsevier), 1992 , vol. 31, # 12 p. 4119 - 4124 |
|
~% 
5353-15-1 |
| Literature: Shulgrin,A.T. Canadian Journal of Chemistry, 1965 , vol. 43, p. 3437 - 3440 |
| Precursor 8 | |
|---|---|
| DownStream 5 | |
| HS Code | 2909309090 |
|---|---|
| Summary | 2909309090 other aromatic ethers and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |