| Description |
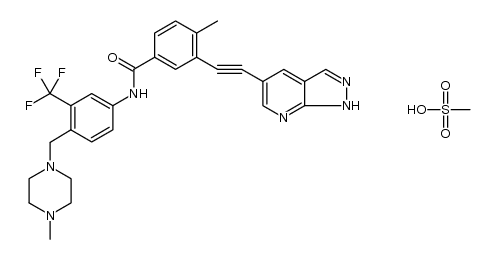
GZD824 dimesylate (HQP1351 dimesylate) is an orally bioavailable pan-Bcr-Abl inhibitor with potency against a wide range of Bcr-Abl mutants and the native enzyme (IC50=0.34 nM). GZD824 dimesylate has antitumor activity[1].
|
| Related Catalog |
|
| Target |
IC50: 0.68 nM (Bcr-AblT315I), 0.27 nM (Bcr-AblE255K) , 0.71 nM (Bcr-AblG250E) , 0.15 nM (Bcr-AblQ252H), 0.35 nM (Bcr-Abl H396P), 0.29 nM (Bcr-Abl M351T), 0.35 nM (Bcr-AblY253F), Bcr-AblF317L[1]
|
| In Vitro |
GZD824 dimesylate shows antiproliferative activity in stably transformed Ba/F3 cells whose growth was driven by native Bcr-Abl or Bcr-Abl mutants[1]. GZD824 dimesylate selectively and potently inhibits the proliferation of Bcr-Abl-positive leukemia cells[1]. GZD824 dimesylate inhibits Bcr-Abl signaling in K562 (1-20 nM; 4.0 hours) and Ba/F3 stable cell lines expressing native Bcr-Abl (0.1-100 nM; 4.0 hours) or Bcr-AblT315I(0.1-100 nM; 4.0 hours)[1]. Western Blot Analysis[1] Cell Line: K562 cells Concentration: 1 nM, 2 nM, 5 nM, 10 nM, 20nM Incubation Time: 4.0 hours Result: Inhibited Bcr-Abl signaling in K562 cell lines.
|
| In Vivo |
GZD824 dimesylate suppresses tumor growth in mice bearing allografted Ba/F3 cells expressing Bcr-Abl WT[1]. GZD824 dimesylate (1-20 mg/kg; i.g.; daily; for 10 days) significantly increases the median survival of the mice bearing allografted Ba/F3 cells expressing Bcr-AblT315I[1]. GZD824 dimesylate exhibits a good oral bioavailability (rat 48.7%) and Cmax (i.v. 1375.6 μg/L; oral 390.5 μg/L) following administration (5 mg/kg for i.v.; 25 mg/kg for oral) in rat[1]. Animal Model: SCID nude mice, bearing allografted Ba/F3 cells expressing Bcr-AblT315I[1] Dosage: 1 mg/kg, 2 mg/kg, 5.0 mg/kg, 10 mg/kg, 20 mg/kg Administration: Oral gavage, daily, for 10 days Result: Efficiently prolongs animal survival in an allograft leukemia tumor model. Animal Model: Rats[1] Dosage: 5 mg/kg for i.v.; 25 mg/kg for oral (Pharmacokinetic Analysis) Administration: Intravenous injection and oral administration Result: Oral bioavailability (48.7%), Cmax(1375.6 μg/L for i.v.; 390.5μg/L for oral), T1/2 (5.6 hour for i.v.; 10.6 hours for oral).
|
| References |
[1]. Ren X, Pan X, Zhang Z, Identification of GZD824 as an orally bioavailable inhibitor that targets phosphorylated and nonphosphorylated breakpoint cluster region-Abelson (Bcr-Abl) kinase and overcomes clinically acquired mutation-induced resistance against imatinib. J Med Chem. 2013 Feb 14;56(3):879-94.
|