151767-02-1
| Name | Montelukast sodium |
|---|---|
| Synonyms |
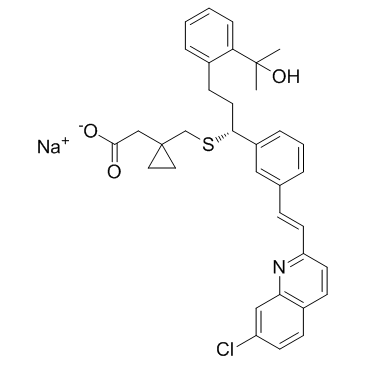
Cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]-, sodium salt (1:1)
Montelukast SodiuM Hydrate Montelukast sodium {1-[({(1R)-1-{3-[(E)-2-(7-chloroquinoléin-2-yl)éthényl]phényl}-3-[2-(1-hydroxy-1-méthyléthyl)phényl]propyl}sulfanyl)méthyl]cyclopropyl}acétate de sodium cyclopropaneacetic acid, 1-[[[(1R)-1-[3-[(E)-2-(7-chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]-, monosodium salt Sodium {1-[({(1R)-1-{3-[(E)-2-(7-chloroquinolin-2-yl)vinyl]phenyl}-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetate Natrium-{1-[({(1R)-1-{3-[(E)-2-(7-chlorchinolin-2-yl)ethenyl]phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetat [R-(E)]-1-[[[1-[3-[2-(7-Chloro-2-quinolinyl)ethenyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic Acid Monosodium Salt sodium {1-[({(1R)-1-{3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetate sodium,2-[1-[[(1R)-1-[3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl]-3-[2-(2-hydroxypropan-2-yl)phenyl]propyl]sulfanylmethyl]cyclopropyl]acetate Montelukast sodium salt Sodium {1-[({(1R)-1-{3-[(E)-2-(7-chloro-2-quinolinyl)vinyl]phenyl}-3-[2-(2-hydroxy-2-propanyl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetate Sodium 1-((((R)-m-((E)-2-(7-Chloro-2-quinolyl)vinyl)-a-(o-(1-hydroxy-1-methylethyl)phenethyl)benzyl)thio)methyl)cyclopropaneacetate Montelukast monosodium salt sodium {1-[({(1R)-1-{3-[(E)-2-(7-chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl}sulfanyl)methyl]cyclopropyl}acetate Singulair Montelukast (sodium) |
| Description | Montelukast sodium is a potent, selective CysLT1 receptor antagonist. |
|---|---|
| Related Catalog | |
| Target |
CysLT1 Autophagy |
| In Vitro | Montelukast may contribute to the reduction of eosinophilic inflammation in upper-airway inflammatory diseases such as rhinitis and nasal polyposis. Montelukast has a significant inhibitory effect on FBS-induced GM-CSF, IL-6, and IL-8 secretion, but not sICAM-1, in nasal mucosa and polyp epithelial cells. Montelukast also shows an inhibitory effect (p<0.05) on ECM-induced eosinophil survival from both nasal mucosa and polyp epithelial cells[1]. |
| In Vivo | Montelukast significantly reduces mild, moderate, and part of severe exacerbations in chronic mild to moderate asthma, but it has inferior efficacy to ICS or ICS plus LABA[2]. Rats with induced asthma have up-regulated NK1R expression in the airway, and montelukast can down regulate NK1R expression during airway remodeling[3]. Blockade of CysLT1R by repeated treatment with montelukast (1 or 2 mg/kg, ig, 4 weeks) reduces Aβ1-42-induced CysLT1R expression and also suppresses Aβ1-42-induced increments of NF-κB p65, TNF-α, IL-1β and caspase-3 activation, and Bcl-2 downregulation in the hippocampus and cortex. Correspondingly, montelukast treatment significantly improves Aβ1-42-induced memory impairment in mice, but has little effect on normal mice[4]. |
| Cell Assay | Nasal mucosa and polyp epithelial cells are stimulated with fetal bovine serum (FBS) with or without MK for 24 hours, and cytokine concentrations in epithelial secretions are measured by ELISA. After incubating peripheral blood eosinophils with epithelial cell-conditioned media (ECM) with or without montelukast up to 3 days, eosinophil survival is assessed by Trypan blue dye exclusion[1]. |
| Animal Admin | Rats: Twenty four Sprague Dawley rats are randomLy divided into control group, asthma, and montelukast group. A rat model of asthma is induced by ovalbumin (OVA) inhalation. Normal saline is used instead of sensitizing solution and 1% OVA in the control group. Each rat in the montelukast group is given montelukast (15mg/kg) by gavage 2h before OVA inhalation. All rats are treated for 8 weeks[3]. Mice: Montelukast is dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na). Mice are randomLy assigned to 4 groups: (1) vehicle plus vehicle,(2) Aβ1-42 plus vehicle, (3) Aβ1-42 plus montelukast (1.0 mg/kg), (4) Aβ1-42 plus montelukast (2.0 mg/kg). The solutions are injected bilaterally into the cerebroventricles through the micropipette[4]. |
| References |
| Boiling Point | 750.5ºC at 760mmHg |
|---|---|
| Melting Point | 115 °C(dec.) |
| Molecular Formula | C35H35ClNNaO3S |
| Molecular Weight | 608.165 |
| Flash Point | 407.7ºC |
| Exact Mass | 607.192383 |
| PSA | 98.55000 |
| LogP | 7.61330 |
| Appearance | white to tan |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
| Water Solubility | DMSO: ≥8mg/mL at 60°C |
|
Montelukast Sodium Hydrate
Revision number: 5
SAFETY DATA SHEET Section1. IDENTIFICATION Product name:Montelukast Sodium Hydrate Revision number:5 Section2. HAZARDS IDENTIFICATION GHS classification PHYSICAL HAZARDSNot classified Not classified HEALTH HAZARDS ENVIRONMENTAL HAZARDSNot classified GHS label elements, including precautionary statements Pictograms or hazard symbolsNone No signal word Signal word Hazard statementsNone None Precautionary statements: Section3. COMPOSITION/INFORMATION ON INGREDIENTS Substance/mixture:Substance Components:Montelukast Sodium Hydrate Percent:>98.0%(LC)(T) CAS Number:151767-02-1 Chemical Formula:C35H35ClNNaO3S·xH2O Section4. FIRST AID MEASURES Inhalation:Remove victim to fresh air and keep at rest in a position comfortable for breathing. Get medical advice/attention if you feel unwell. Skin contact:Remove/Take off immediately all contaminated clothing. Rinse skin with water/shower. If skin irritation or rash occurs: Get medical advice/attention. Eye contact:Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Ingestion:Get medical advice/attention if you feel unwell. Rinse mouth. A rescuer should wear personal protective equipment, such as rubber gloves and air- Protection of first-aiders: tight goggles. Section5. FIRE-FIGHTING MEASURES Suitable extinguishingDry chemical, foam, water spray, carbon dioxide. media: Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to from the chemical:generate poisonous fume. Montelukast Sodium Hydrate Section5. FIRE-FIGHTING MEASURES Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing method according to the surrounding situation is used. Uninvolved persons should evacuate to a safe place. In case of fire in the surroundings: Remove movable containers if safe to do so. Special protectiveWhen extinguishing fire, be sure to wear personal protective equipment. equipment for firefighters: Section6. ACCIDENTAL RELEASE MEASURES Use personal protective equipment. Keep people away from and upwind of spill/leak. Personal precautions, protective equipment and Entry to non-involved personnel should be controlled around the leakage area by emergency procedures: roping off, etc. Environmental precautions: Prevent product from entering drains. Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it. containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with up: appropriate laws and regulations. Section7. HANDLING AND STORAGE Precautions for safe handling Handling is performed in a well ventilated place. Wear suitable protective equipment. Technical measures: Prevent dispersion of dust. Wash hands and face thoroughly after handling. Use a local exhaust if dust or aerosol will be generated. Advice on safe handling: Avoid contact with skin, eyes and clothing. Conditions for safe storage, including any incompatibilities Storage conditions:Keep container tightly closed. Store in a cool and dark place. Store under inert gas. Protect from moisture. Store away from incompatible materials such as oxidizing agents. Light-sensitive, Hygroscopic Packaging material:Comply with laws. Section8. EXPOSURE CONTROLS / PERSONAL PROTECTION Engineering controls:Install a closed system or local exhaust as possible so that workers should not be exposed directly. Also install safety shower and eye bath. Personal protective equipment Respiratory protection: Dust respirator. Follow local and national regulations. Hand protection:Protective gloves. Safety glasses. A face-shield, if the situation requires. Eye protection: Skin and body protection: Protective clothing. Protective boots, if the situation requires. Section9. PHYSICAL AND CHEMICAL PROPERTIES Physical state (20°C):Solid Crystal- Powder Form: Colour:White - Almost white No data available Odour: pH: 9.7 (1% aq soln.) Melting point/freezing point:115°C (dec.) Boiling point/range:No data available No data available Flash point: Flammability or explosive limits: Lower:No data available No data available Upper: Relative density:No data available Solubility(ies): [Water]Very soluble Montelukast Sodium Hydrate Section9. PHYSICAL AND CHEMICAL PROPERTIES [Other solvents] Very soluble:Methanol, Ethanol Insoluble:Acetonitrile Section10. STABILITY AND REACTIVITY Chemical stability:Stable under proper conditions. Possibility of hazardous No special reactivity has been reported. reactions: Incompatible materials: Oxidizing agents Hazardous decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulfur oxides, Hydrogen chloride products: Section11. TOXICOLOGICAL INFORMATION Acute Toxicity:ipr-mus TDLo:1 mg/kg Skin corrosion/irritation: No data available Serious eyeNo data available damage/irritation: Germ cell mutagenicity: No data available Carcinogenicity: IARC =No data available No data available NTP = Reproductive toxicity:No data available RTECS Number:GZ0698000 Section12. ECOLOGICAL INFORMATION Ecotoxicity: Fish:No data available No data available Crustacea: Algae:No data available Persistence / degradability: No data available BioaccumulativeNo data available potential(BCF): Mobility in soil Log Pow:No data available Soil adsorption (Koc):No data available Henry's LawNo data available constant(PaM3/mol): Section13. DISPOSAL CONSIDERATIONS Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when disposing of the substance. Section14. TRANSPORT INFORMATION Hazards Class:Does not correspond to the classification standard of the United Nations UN-No:Not listed Section15. REGULATORY INFORMATION Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002 and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were prescribed. Montelukast Sodium Hydrate SECTION 16 - ADDITIONAL INFORMATION N/A |
| Symbol |


GHS05, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318-H361fd |
| Precautionary Statements | P280-P305 + P351 + P338 + P310 |
| Hazard Codes | Xi |
| Risk Phrases | 63-41-62 |
| Safety Phrases | 26 |
| RIDADR | NONH for all modes of transport |
| RTECS | GZ0698000 |
| HS Code | 2933499090 |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

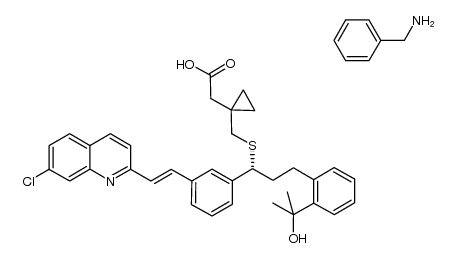
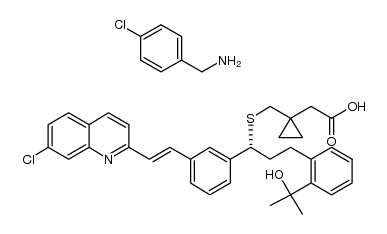


![1-[[[(1R)-1-[3-[(1S)-1-[[[1-(Carboxymethyl)cyclopropyl]methyl]thio]-2-(7-chloro-2-quinolinyl)ethyl]phenyl]-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl]thio]methyl]cyclopropaneacetic acid structure](https://image.chemsrc.com/caspic/331/1187586-58-8.png)