111857-96-6
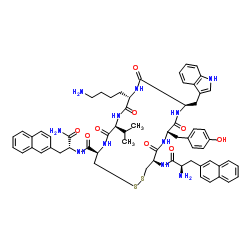
| Name | h-d-2-nal-cys-tyr-d-trp-lys-val-cys-2-nal-nh2 |
|---|---|
| Synonyms | (4R,7S,10S,13R,16S,19R)-10-(4-Aminobutyl)-N-[(2R)-1-amino-3-(2-naphthyl)-1-oxo-2-propanyl]-19-{[(2R)-2-amino-3-(2-naphthyl)propanoyl]amino}-16-(4-hydroxybenzyl)-13-(1H-indol-3-ylmethyl)-7-isopropyl-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosane-4-carboxamide |
| Description | BIM 23042, a certain somatostatin (SS) octapeptide analogue, is a selective neuropeptide neuromedin B receptor (NMB-R, BB1) antagonist. BIM 23042 has 100-fold lower affinity for gastrin-releasing peptide (GRP) receptor (BB2). BIM 23042 inhibits Neuromedin B (HY-P0241), ICI 216140 and DPDM-bombesin ethylamide-induced Ca2+ release[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | BIM 23042 (5 μM) 在 huNMBR 细胞中竞争性抑制 Neuromedin B 诱导的 [3H] 花生四烯酸释放,Ki 为 49 nM[1]。 |
| In Vivo | BIM 23042 (10μg; 静脉给药; 单次推注) 可减轻神经源性肿胀和热和机械致敏[2]。 Animal Model: Mice were 25-30 g male C57BL/6[2] Dosage: 10 μg Administration: IV; a single bolus Result: Attenuated neurogenic swelling and thermal and mechanical sensitization. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 1554.2±65.0 °C at 760 mmHg |
| Molecular Formula | C62H73N11O9S2 |
| Molecular Weight | 1192.452 |
| Flash Point | 893.7±34.3 °C |
| Exact Mass | 1191.503418 |
| PSA | 412.45000 |
| LogP | 6.00 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.709 |