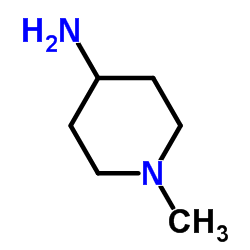
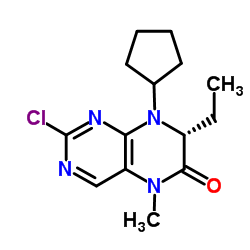
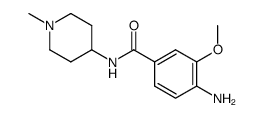
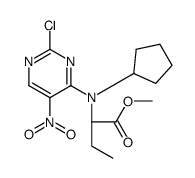
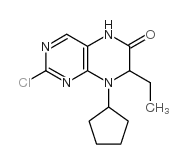
755038-02-9
| Name | 4-[[(7R)-8-cyclopentyl-7-ethyl-5-methyl-6-oxo-7H-pteridin-2-yl]amino]-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide |
|---|---|
| Synonyms |
BI2536
BI-2536 4-[[(7R)-8-cyclopentyl-7-ethyl-5,6,7,8-tetrahydro-5-methyl-6-oxo-2-pteridinyl]amino]-3-methoxy-N-(1-methyl-4-piperidinyl)-benzamide,type I anhydrate BI 2536 4-{[(7R)-8-Cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydro-2-pteridinyl]amino}-3-methoxy-N-(1-methyl-4-piperidinyl)benzamide (R)-4-((8-cyclopentyl-7-ethyl-5-methyl-6-oxo-5,6,7,8-tetrahydropteridin-2-yl)amino)-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide 4-[[(7R)-8-cyclopentyl-7-ethyl-5,6,7,8-tetrahydro-5-methyl-6-oxo-2-pteridinyl]amino]-3-methoxy-N-(1-methyl-4-piperidinyl)-benzamide,type III anhydrate 4-[[(7R)-8-cyclopentyl-7-ethyl-5,6,7,8-tetrahydro-5-methyl-6-oxo-2-pteridinyl]amino]-3-methoxy-N-(1-methyl-4-piperidinyl)-benzamide 4-[[(7R)-8-cyclopentyl-7-ethyl-5,6,7,8-tetrahydro-5-methyl-6-oxo-2-pteridinyl]amino]-3-methoxy-N-(1-methyl-4-piperidinyl)-benzamide,type II anhydrate S1109_Selleck |
| Description | BI 2536 is a dual PLK1 and BRD4 inhibitor with IC50s of 0.83 and 25 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
PLK1:0.83 nM (IC50) Plk2/Snk:3.5 nM (IC50) Plk3/Fnk:9 nM (IC50) BRD4:25 nM (IC50) |
| In Vitro | Exceeding a 100-fold concentration range starting at 10 nM, BI 2536 causes HeLa cells to accumulate with a 4N DNA content, indicative of a cell-cycle block in either G2 phase or mitosis. In addition to HeLa cells, BI 2536 potently inhibits the proliferation of a panel of 32 human cancer cell lines, representing diverse organ derivations (including carcinomas of the breast, colon, lung, pancreas, and prostate, melanomas, and hematopoietic cancers) and varied patterns of tumor suppressor or oncogene mutations (including RB1, TP53, PTEN, andKRAS status). The half-maximal effective concentration (EC50) values in this cell panel ranged 2-25 nM, whereas a concentration of 100 nM of BI 2536 is typically sufficient for inducing a complete mitotic arrest. The proliferation of exponentially growing hTERT-RPE1, human umbilical vein endothelial cells (HUVECs), and normal rat kidney (NRK) cells is blocked at EC50values ranging 12-31 nM, indicating a comparable sensitivity of cycling nontransformed cells to BI 2536[3]. |
| In Vivo | BI 2536 (40-50 mg/kg, i.v.) blocks the growth of human cancer xenografts in immunodeficient, nu/nu mice. Consecutive cycles of 40-50 mg/kg BI 2536 given i.v. once or twice per week are found to be highly efficacious in diverse xenograft models, such as the HCT 116 colon cancer with complete tumor suppression with the twice per week schedule (treated versus the control (T/C) value 0.3%) and a T/C value of 16% with once per week treatment; both schedules are well-tolerated, as judged by clinical signs and absence of major body-weight changes[3]. |
| Kinase Assay | Enzyme activity assays for Plk1, Plk2, and Plk3 are performed in the presence of serially diluted inhibitor (BI 2536) with 20 ng of Recombinant kinase and 10 mg casein from bovine milk as the substrate. Kinase reactions are performed in a final volume of 60 mL for 45 min at 30°C (15 mM MgCl2, 25 mM MOPS [pH 7.0], 1 mM DTT, 1% DMSO, 7.5 mM ATP, 0.3 mCi γ-P33-ATP). Reactions are terminated by the addition of 125 mL of ice-cold 5% TCA. After transfer of the precipitates to Multi-Screen mixed ester cellulose filter plates, plates are washed with1%TCA and quantified radiometrically. Dose-response curves are used for calculating IC50 values[3]. |
| Cell Assay | Cell proliferation assays are performed by incubation in the presence of various concentrations of BI 2536 (10 nM-1 μM) for 72 hr, and cell growth is assessed by the measurement of Alamar Blue dye conversion in a fluorescence spectrophotometer. Effective concentrations at which cellular growth is inhibited by 50% (EC50) are extrapolated from the dose-response curve fit[3]. |
| Animal Admin | Mice[3] Female BomTac:NMRI-Foxn1nu mice are grafted subcutaneously with HCT 116 colon-carcinoma, NCI-H460, or A549 lung-carcinoma cells by subcutaneous injection, respectively, of 2×106, 1×106, and 1×107 cells into the flank of each mouse. When tumors reached a volume of approximately 50 mm3, animals are pair-matched into treatment and control groups of ten mice each. In regression experiments, treatment is not initiated until the mean tumor volume reached 500 mm3. BI 2536 is injected intravenously into the tail vein at the indicated dose and schedule. The administration volume is 10 mL per kg body weight. Tumor volumes are determined three times a week with a caliper. The results are converted to tumor volume (mm3) by the following formula: length×width2×π/6. The weight of the mice is determined as an indicator of tolerability on the same days. For statistical analysis, the treatment group is compared with the vehicle control group in a one-sided (decreasing) exact Wilcoxon test. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Molecular Formula | C28H39N7O3 |
| Molecular Weight | 521.654 |
| Exact Mass | 521.311462 |
| PSA | 102.93000 |
| LogP | 1.81 |
| Index of Refraction | 1.634 |
| Storage condition | -20°C |
|
~% 
755038-02-9 |
| Literature: US2006/35903 A1, ; Page/Page column 21 ; |
|
~% 
755038-02-9 |
| Literature: US2008/177066 A1, ; Page/Page column 57; 60 ; |
|
~62% 
755038-02-9 |
| Literature: Budin, Ghyslain; Yang, Katherine S.; Reiner, Thomas; Weissleder, Ralph Angewandte Chemie - International Edition, 2011 , vol. 50, # 40 p. 9378 - 9381 |
|
~% 
755038-02-9 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 40 p. 9378 - 9381 |
|
~% 
755038-02-9 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 40 p. 9378 - 9381 |
|
~% 
755038-02-9 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 40 p. 9378 - 9381 |
|
~% 
755038-02-9 |
| Literature: Angewandte Chemie - International Edition, , vol. 50, # 40 p. 9378 - 9381 |
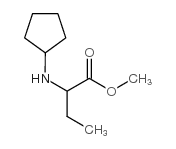
| Precursor 7 | |
|---|---|
| DownStream 0 | |