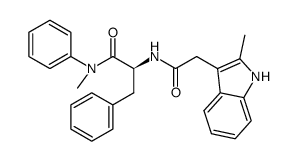
1352879-65-2
| Name | (S)-N-methyl-2-(2-(2-methyl-1H-indol-3-yl)acetamido)-N,3-diphenylpropanamide |
|---|---|
| Synonyms |
pf74
pf-3450074 |
| Description | PF-3450074 (PF-74) is a specifical inhibitor of HIV-1 capsid protein (CA) and displays a broad-spectrum inhibition of HIV isolates with submicromolar potency (EC50=8-640 nM). PF-3450074 (PF-74) acts at an early stage of HIV-1 infection, inhibits viral replication by directly competing with the binding of CPSF6 and NUP153, and blocks the uncoating, assembly, and the reverse transcription steps of the viral life cycle[1][2]. CPSF6: nuclear host factors cleavage and polyadenylation specific factor 6; NUP153: nucleoporin 153. |
|---|---|
| Related Catalog | |
| Target |
HIV-1 (NL4.3 strain):0.72 μM (IC50) |
| In Vitro | PF-3450074 (PF-74) exhibits anti-viral activities against HIV wild type NL4-3 and HIV T107N mutant with EC50 values of 0.72 μM and 4.5μM, respectively[1]. PF-3450074 (PF-74) displays a good potency in primary human peripheral blood mononuclear cells (PBMCs), inhibits HIV-193RW025, HIV-1JR-CSF and HIV-193MW965 with IC50 values of 1.5 ± 0.9 μM; 0.6 ± 0.20 μM; and 0.6 ± 0.10 μM, respectively. This compound shows Median IC50 and CC50 values of 0.9 ± 0.5 μM and 90.5 ± 5.9 μM, respectively[1]. The KD for the interaction between PF-74 and the CA hexamer, derived in the same manner as for NUP153, is determined to be 176 ± 78 nM[1]. PF-3450074 (PF-74) (10 μM; 8 hours) results in a marked reduction in late products of reverse transcription in HeLa-P4 cells with DNase I-treated stocks of Env-defective HIV-1 (R9.Env-)[2]. RT-PCR[2] Cell Line: HeLa-P4 cells Concentration: 10 μM Incubation Time: 8 hours Result: Inhibited HIV-1 reverse transcription in target cells. |
| References |
| Molecular Formula | C27H27N3O2 |
|---|---|
| Molecular Weight | 425.52200 |
| Exact Mass | 425.21000 |
| PSA | 65.20000 |
| LogP | 4.80020 |
| RIDADR | NONH for all modes of transport |
|---|