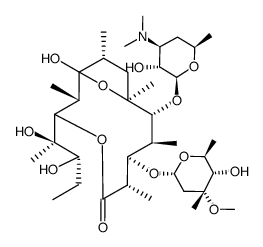
23893-13-2
| Name | Anhydro Erythromycin A |
|---|---|
| Synonyms |
Erythromycin Anhydride,Impurity D
(1S,2R,3R,4S,5R,8R,9S,10S,11R,12R,14R)-11-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5-ethyl-3-hydroxy-9-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahyd ;ro-2H-pyran-2-yl]oxy}-2,4,8,10,12,14-hexamethyl-6,15,16-trioxatricyclo[10.2.1.1]hexadecan-7-one (non-preferred name) Erythromycin,9-deoxo-6,12-dideoxy-6,9:9,12-diepoxy ANHYDROERYTHROMYCIN A EM 202 9-Deoxo-6,12-dideoxy-6,9:9,12-diepoxy-erythromycin Erythromycin anhydride Anhydroerythromycin (1S,2R,3R,4S,5R,8R,9S,10S,11R,12R,14R)-11-{[(2S,3R,4S,6R)-4-(Dimethylamino)-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5-ethyl-3-hydroxy-9-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahyd ;ro-2H-pyran-2-yl]oxy}-2,4,8,10,12,14-hexamethyl-6,15,16-trioxatricyclo[10.2.1.1]hexadecan-7-one 6,9,12-Anhydroerythromycin A 6,9:9,12-Diepoxy-9-deoxo-6,12-dideoxyerythromycin |
| Description | Anhydroerythromycin A is a degradation product of the macrolide antibiotic erythromycin. Anhydroerythromycin A is formed via degradation of erythromycin in acidic aqueous solutions in vitro as well as in vivo. Anhydroerythromycin A is active against S. aureus and B. cereus in vitro (MICs = 12.5 and 6.25 μg/ml, respectively). Anhydroerythromycin A also inhibits steroid 6β-hydroxylase activity associated with the cytochrome P450 (CYP) isoform CYP3A in human liver microsomes. |
|---|
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 787.2±60.0 °C at 760 mmHg |
| Melting Point | 125-127ºC |
| Molecular Formula | C37H65NO12 |
| Molecular Weight | 715.911 |
| Flash Point | 429.9±32.9 °C |
| Exact Mass | 715.450684 |
| PSA | 154.84000 |
| LogP | 3.93 |
| Vapour Pressure | 0.0±6.2 mmHg at 25°C |
| Index of Refraction | 1.540 |
| Storage condition | −20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 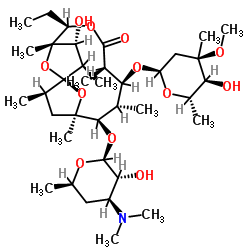
23893-13-2 |
| Literature: Journal of Organic Chemistry, , vol. 52, # 6 p. 990 - 996 |
|
~% 
23893-13-2 |
| Literature: Kibwage, Isaac O.; Busson, Roger; Janssen, Gerard; Hoogmartens, Jos; Vanderhaeghe, Hubert Journal of Organic Chemistry, 1987 , vol. 52, # 6 p. 990 - 996 |
|
~% 
23893-13-2 |
| Literature: Journal of Organic Chemistry, , vol. 52, # 6 p. 990 - 996 |
|
~% 
23893-13-2 |
| Literature: Journal of Organic Chemistry, , vol. 52, # 6 p. 990 - 996 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |