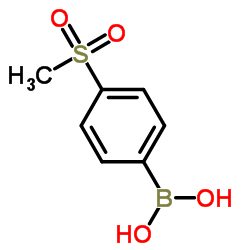
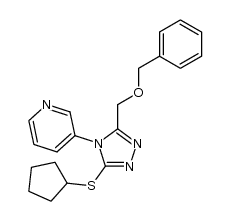
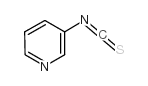
1418013-75-8
| Name | 3-[3-(Cyclopentylsulfanyl)-5-({[2-methyl-4'-(methylsulfonyl)-4-bi phenylyl]oxy}methyl)-4H-1,2,4-triazol-4-yl]pyridine |
|---|---|
| Synonyms |
3-[3-(Cyclopentylsulfanyl)-5-({[2-methyl-4'-(methylsulfonyl)-4-biphenylyl]oxy}methyl)-4H-1,2,4-triazol-4-yl]pyridine
NMS-873 |
| Description | NMS-873 is a potent, selective allosteric VCP/p97 inhibitor with IC50 value of 30 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 30 nM[1] |
| In Vitro | NMS-873 has antiproliferative effect on a panel of tumor cell lines with IC50 values in the range of 0.08 μM to 2 μM. For HCT116 and HeLa cells, the IC50 values are 0.4 μM and 0.7 μM, respectively. NMS-873 reduces VCP sensitivity to trypsin digestion, preventing degradation of the linker-D2 domain. NMS-873 induces clear, dose-dependent accumulation of poly-Ub proteins and stabilization of cyclin E and Mcl-1 at doses consistent with its antiproliferative IC50 value[1]. |
| Kinase Assay | The ATPase activity and the kinetic parameters of recombinant wild-type VCP and its mutants are evaluated by monitoring ADP formation in the reaction, using a modified NADH-coupled assay46. As ADP and NADH are ATP-competitive inhibitors of VCP ATPase activity, the standard protocol for the NADH-coupled assay is modified into a two-step procedure. In the first part, an ATP-regenerating system (40 U/mL pyruvate kinase and 3 mM phosphoenolpyruvate) recycles the ADP produced by VCP activity, keeps the substrate concentration constant (thus preventing product inhibition) and accumulates a stoichiometric amount of pyruvate. In the second part, the VCP enzymatic reaction is quenched with 30 mM EDTA and 250 μM NADH and stoichiometrically oxidized by 40 U/mL lactic dehydrogenase to reduce accumulated pyruvate. The decrease of NADH concentration is measured at 340 nm using a Tecan Safire 2 reader plate. The assay is performed in 96- or 384-well UV platesin a reaction buffer with 50 mM Hepes, pH 7.5, 0.2 mg/mL BSA, 10 mM MgCl2 and 2 mM DTT. |
| Cell Assay | Cells are seeded at 1,600 cells per well in 384-well white clear-bottom plates. Twenty-four hours after seeding, cells are treated with the compounds (eight dilution points, in duplicate, for each compound) and incubated for an additional 72 h at 37°C under a 5% CO2 atmosphere. Cells are then lysed, and the ATP content in each well is determined using a thermostable firefly luciferase-based assay from Promega as a measure of cell viability. IC50 values are calculated using the percentage of growth of treated cells versus the untreated control. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 769.3±70.0 °C at 760 mmHg |
| Molecular Formula | C27H28N4O3S2 |
| Molecular Weight | 520.666 |
| Flash Point | 419.0±35.7 °C |
| Exact Mass | 520.160278 |
| PSA | 120.65000 |
| LogP | 4.77 |
| Appearance | white to beige |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.675 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble25mg/mL, clear |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Hazard Codes | Xn |
| Risk Phrases | 22 |
| RIDADR | NONH for all modes of transport |
|
~39% 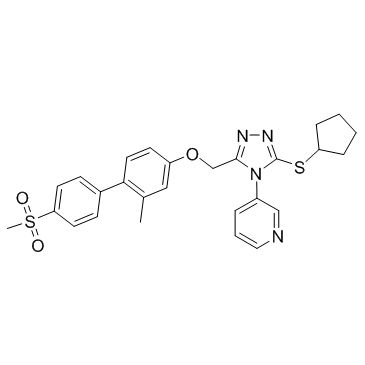
1418013-75-8 |
| Literature: Polucci, Paolo; Magnaghi, Paola; Angiolini, Mauro; Asa, Daniela; Avanzi, Nilla; Badari, Alessandra; Bertrand, Jay; Casale, Elena; Cauteruccio, Silvia; Cirla, Alessandra; Cozzi, Liviana; Galvani, Arturo; Jackson, Peter K.; Liu, Yichin; Magnuson, Steven; Malgesini, Beatrice; Nuvoloni, Stefano; Orrenius, Christian; Sirtori, Federico Riccardi; Riceputi, Laura; Rizzi, Simona; Trucchi, Beatrice; O'Brien, Tom; Isacchi, Antonella; Donati, Daniele; D'Alessio, Roberto Journal of Medicinal Chemistry, 2013 , vol. 56, # 2 p. 437 - 450 |
|
~% 
1418013-75-8 |
| Literature: Polucci, Paolo; Magnaghi, Paola; Angiolini, Mauro; Asa, Daniela; Avanzi, Nilla; Badari, Alessandra; Bertrand, Jay; Casale, Elena; Cauteruccio, Silvia; Cirla, Alessandra; Cozzi, Liviana; Galvani, Arturo; Jackson, Peter K.; Liu, Yichin; Magnuson, Steven; Malgesini, Beatrice; Nuvoloni, Stefano; Orrenius, Christian; Sirtori, Federico Riccardi; Riceputi, Laura; Rizzi, Simona; Trucchi, Beatrice; O'Brien, Tom; Isacchi, Antonella; Donati, Daniele; D'Alessio, Roberto Journal of Medicinal Chemistry, 2013 , vol. 56, # 2 p. 437 - 450 |
|
~% 
1418013-75-8 |
| Literature: Polucci, Paolo; Magnaghi, Paola; Angiolini, Mauro; Asa, Daniela; Avanzi, Nilla; Badari, Alessandra; Bertrand, Jay; Casale, Elena; Cauteruccio, Silvia; Cirla, Alessandra; Cozzi, Liviana; Galvani, Arturo; Jackson, Peter K.; Liu, Yichin; Magnuson, Steven; Malgesini, Beatrice; Nuvoloni, Stefano; Orrenius, Christian; Sirtori, Federico Riccardi; Riceputi, Laura; Rizzi, Simona; Trucchi, Beatrice; O'Brien, Tom; Isacchi, Antonella; Donati, Daniele; D'Alessio, Roberto Journal of Medicinal Chemistry, 2013 , vol. 56, # 2 p. 437 - 450 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |