106577-39-3
| Name | Hederacolchiside A1 |
|---|---|
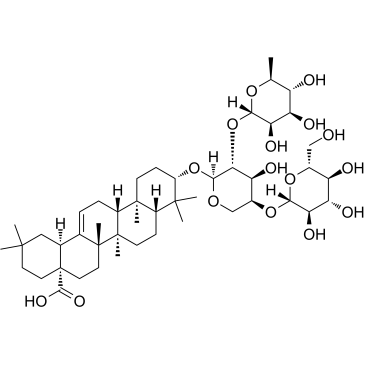
| Synonyms | (3β,5ξ,18α)-3-{[6-Deoxy-α-L-mannopyranosyl-(1->2)-[β-D-glucopyranosyl-(1->4)]-α-L-arabinopyranosyl]oxy}olean-12-en-28-oic acid |
| Description | Hederacolchiside A1, isolated from Pulsatilla chinensis, suppresses proliferation of tumor cells by inducing apoptosis through modulating PI3K/Akt/mTOR signaling pathway[1]. Hederacolchiside A1 has antischistosomal activity, affecting parasite viability both in vivo and in vitro[2]. |
|---|---|
| Related Catalog | |
| Target |
PI3K Akt mTOR |
| In Vitro | Hederacolchiside A1 reduces the mitochondrial membrane potential and Bcl-2 protein levels, whereas cleaved caspase-3 was higher[1]. Hederacolchiside A1 effectively inhibits the phosphorylations of phosphatidylinositol 3 kinase (PI3K), protein kinase B (Akt), and mammalian target of rapamycin (mTOR) [1]. |
| In Vivo | hederacolchiside A1 (3.0, 4.5, and 6.0 mg/kg, ip) can significantly inhibit the weight of tumor in an H22 xenograft model[1]. Hederacolchiside A1 (3.25, 7.5, and 15.0 mg/kg, ig) can significantly inhibit the weight of tumor in nude mice xenograft tumor models using human breast carcinoma MCF-7 cells[1]. |
| References |
| Density | 1.36±0.1 g/cm3 (20 ºC 760 Torr) |
|---|---|
| Boiling Point | 967.2±65.0 °C at 760 mmHg |
| Melting Point | 253-255℃ (methanol , water ) |
| Molecular Formula | C47H76O16 |
| Molecular Weight | 897.097 |
| Flash Point | 276.2±27.8 °C |
| Exact Mass | 896.513306 |
| LogP | 7.36 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.610 |
| Storage condition | 2-8℃ |
| Water Solubility | Insuluble (3.9E-4 g/L) (25 ºC) |