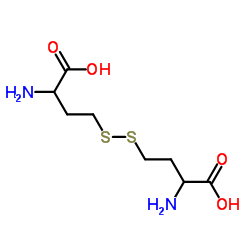
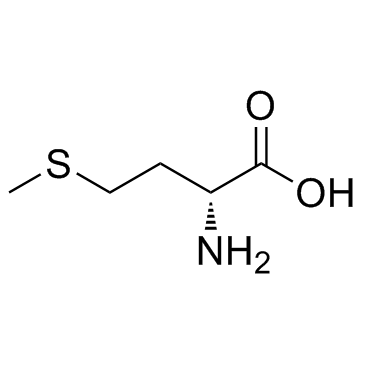
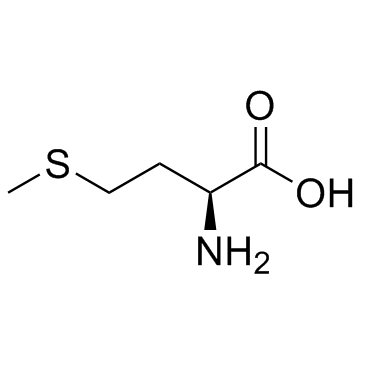
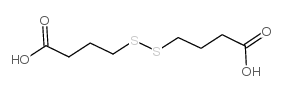
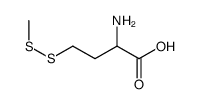
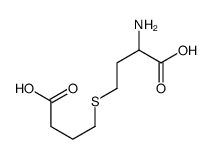
454-29-5
| Name | DL-Homocysteine |
|---|---|
| Synonyms |
DL-HoMocysteine
DL-2-amino-4-mercapto-Butyric acid D-L-homocysteine usafb-12 DL-2-Amino-4-mercaptobutyric acid 2-Amino-4-mercaptobutyric acid D,L-Homocysteine 2-Amino-4-mercaptobutanoic acid HOMOCYSTEINE,DL 2-AMINO-4-MERCAPTOBUYRIC ACID 1-carboxy-3-mercaptopropylamine 2-amino-4-mercapto-DL-Butyric acid 2-amino-4-sulfanylbutanoic acid Homocysteine (VAN) TOTALHOMOCYSTEINE H-DL-Hcys-OH 2-Amino-4-Mercapto-Butyric Acid EINECS 207-222-9 homocystine (±)-Homocysteine 2-amino-4-mercapto-Butanoic acid UNII:S7IJP4A89K MFCD00004898 Homocysteine 2-thioethylglycine Butyric acid, 2-amino-4-mercapto-, DL- (9CI) |
| Description | DL-Homocysteine is a weak neurotoxin, and can affect the production of kynurenic acid in the brain. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | DL-Homocysteine (0.1-0.5 mM) significantly enhances kynurenic acid (KYNA) production in rat cortical slices, and diministes the production of at 3.0, 5.0, and 10.0 mM, with the estimated IC50 of 6.4 (5.5-7.5) mM. DL-Homocysteine dose-dependently inhibits kynurenine aminotransferases I (KATI) activity at concentrations ≥0.2 mM, with an IC50 of 0.566 (0.442-0.724) mM, and the activity of KAT II with IC50 value of 8.046 (5.804-11.154) mM[1]. |
| In Vivo | DL-Homocysteine (1.3 mmol/kg, i.p.) increases KYNA content (pmol/g tissue) from 8.47 ± 1.57 to 13.04 ± 2.86 and 11.4 ± 1.72 in cortex, and from 4.11 ± 1.54 to 10.02 ± 3.08 in rat hippocampus[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 299.7±35.0 °C at 760 mmHg |
| Melting Point | 232-233 °C(lit.) |
| Molecular Formula | C4H9NO2S |
| Molecular Weight | 135.185 |
| Flash Point | 135.0±25.9 °C |
| Exact Mass | 135.035400 |
| PSA | 102.12000 |
| LogP | 0.22 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.538 |
| Storage condition | 2-8°C |
| Stability | Stable. Combustible. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | C |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MT0175000 |
| HS Code | 2930909090 |
|
~36% 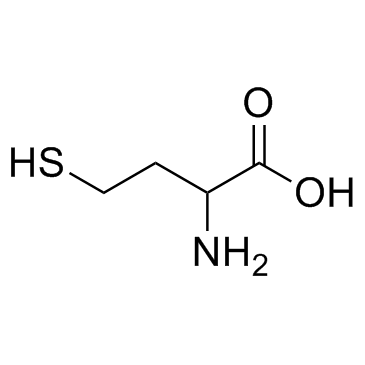
454-29-5 |
| Literature: ADISSEO FRANCE S.A.S.; Huet, Robert; Joerger, Jean-Michel; Henryon, Vivien Patent: US2013/184474 A1, 2013 ; Location in patent: Paragraph 0031; 0032; 0033; 0034; 0035; 0036 ; |
|
~% 
454-29-5 |
| Literature: Allen; Steinman Journal of the American Chemical Society, 1952 , vol. 74, p. 3932 |
|
~% 
454-29-5 |
| Literature: Riegel; du Vigneaud Journal of Biological Chemistry, 1935 , vol. 112, p. 149,153 Full Text Show Details duVigneaud; Brown Biochemical Preparations, 1957 , vol. 5, p. 96 |
|
~% 
454-29-5 |
| Literature: Bregant, Sarah; Burlina, Fabienne; Chassaing, Gerard Bioorganic and Medicinal Chemistry Letters, 2002 , vol. 12, # 7 p. 1047 - 1050 Title/Abstract Full Text View citing articles Show Details Guerard, Christine; Breard, Maud; Courtois, Fabienne; Drujon, Thierry; Ploux, Olivier Bioorganic and Medicinal Chemistry Letters, 2004 , vol. 14, # 7 p. 1661 - 1664 |
|
~% 
454-29-5 |
| Literature: Bommarius, Andreas S.; Drauz, Karlheinz; Guenther, Kurt; Knaup, Guenter; Schwarm, Michael Tetrahedron Asymmetry, 1997 , vol. 8, # 19 p. 3197 - 3200 |
| Precursor 5 | |
|---|---|
| DownStream 9 | |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |