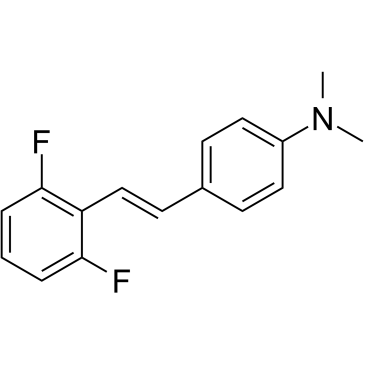
1266684-01-8
| Name | FIDAS-3 |
|---|
| Description | FIDAS-3 is a stilbene derivative and is a potent Wnt inhibitor with an IC50 of 4.9 μM for methionine S-adenosyltransferase 2A (MAT2A). FIDAS-3 effectively competes against S-adenosylmethionine (SAM) for MAT2A binding. FIDAS-3 has anticancer activities[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 4.9 μM (Methionine S-adenosyltransferase 2A (MAT2A))[1] |
| In Vitro | FIDAS-3 (3 μM; 7 days; LS174T cells) treatment significantly inhibits the proliferation of LS174T cells[1]. FIDAS-3 (3-10 μM) treatment inhibits the expression of c-Myc and cyclinD1 in LS174T CRC cells. And FIDAS-3 induces the expression of cell cycle inhibitor, p21WAF1/CIP1[1]. FIDAS-3 (10 μM; 36 h) treatment reduces the levels of both S-adenosylmethionine (SAM) and S-adenosylhomocysteine (SAH) in LS174T cells[1]. Cell Viability Assay[1] Cell Line: LS174T colorectal cancer (CRC) cells Concentration: 3 μM Incubation Time: 7 days Result: Significantly inhibited the proliferation of LS174T cells. |
| In Vivo | FIDAS-3 (20 mg/kg; intraperitoneal injection; daily; for one months; C57BL/6J athymic nude mice) treatment significantly inhibits the growth of xenograft tumors[2]. Animal Model: C57BL/6J athymic nude mice (6-8 week) injected with LS174 cells[2] Dosage: 20 mg/kg Administration: Intraperitoneal injection; daily; for one months Result: Significantly inhibited the growth of xenograft tumors. |
| References |
| Molecular Formula | C16H15F2N |
|---|---|
| Molecular Weight | 259.29 |