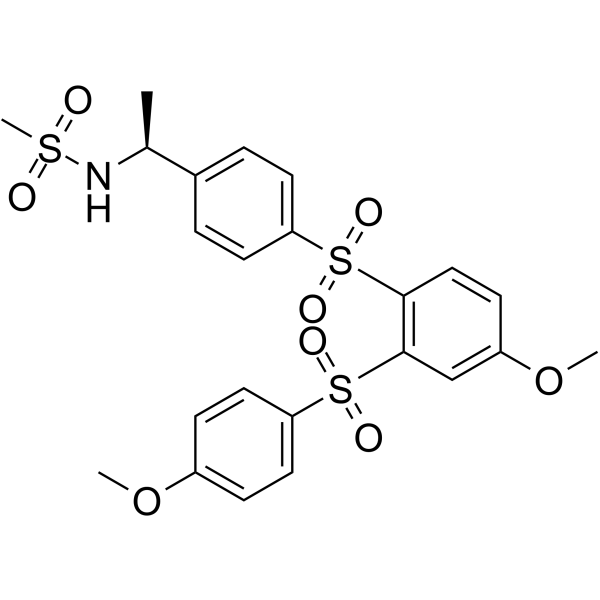
447459-51-0
| Name | N-{(1S)-1-[4-({4-Methoxy-2-[(4-methoxyphenyl)sulfonyl]phenyl}sulfonyl)phenyl]ethyl}methanesulfonamide |
|---|---|
| Synonyms | N-{(1S)-1-[4-({4-Methoxy-2-[(4-methoxyphenyl)sulfonyl]phenyl}sulfonyl)phenyl]ethyl}methanesulfonamide |
| Description | SCH 336 is a potent, selective, inverse and orally active CB2 agonist. SCH 336 inhibits BaF3/CB2 migration. SCH 336 significantly inhibits the migration of leukocytes in vivo. SCH 336 blocks ovalbumin-induced lung eosinophilia in mice[1]. |
|---|---|
| Related Catalog | |
| Target |
hCB2-R |
| In Vitro | SCH 336 (Sch.336) (0-10 µM) competes with [3H]CP55,940 for binding to human CB2 on Sf9 cell membranes with Ki of 1.8 nM, and decreases GTPγS binding on human CB2-containing membranes with an EC50 of 2 nM, decreases potency on CB1-containing membranes with EC50 of 200 nM[1]. SCH 336 inhibits BaF3/CB2 migration to 100 nM 2-AG with an IC50 of 34 nM[1]. |
| In Vivo | SCH 336 (0.02, 0.2, 2.0 mg/kg; i.p.) significantly inhibits the migration of leukocytes[1]. Animal Model: Female B6D2/F1 mice (HU210)[1] Dosage: 0.02, 0.2, 2.0 mg/kg Administration: I.p. Result: Significantly inhibited the migration of leukocytes into the CCL2-soaked gel foam sponge. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 756.6±70.0 °C at 760 mmHg |
| Molecular Formula | C23H25NO8S3 |
| Molecular Weight | 539.64 |
| Flash Point | 411.4±35.7 °C |
| Exact Mass | 539.074219 |
| LogP | 3.91 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.589 |