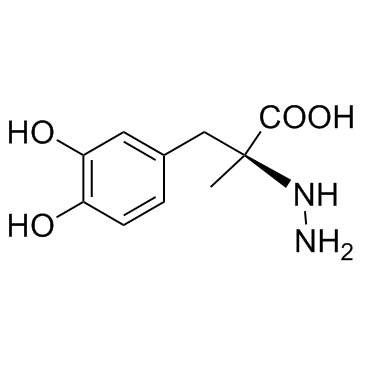
28860-95-9
| Name | carbidopa (anhydrous) |
|---|---|
| Synonyms |
Benzenepropanoic acid, α-hydrazinyl-3,4-dihydroxy-α-methyl-, (αS)-, hydrate (1:1)
Carbidopa (S)-Carbidopa 1-a-(3,4-Dihydroxybenzyl)-a-hydrazinopropionic Acid MFCD00069231 L-a-Methyldopahydrazine (aS)-a-Hydrazino-3,4-dihydroxy-a-methylbenzenepropanoic Acid a-Methyldopahydrazine Lodosin EINECS 249-271-9 carbidopa [INN_en] (S)-(-)-Carbidopa HMD (2S)-3-(3,4-Dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid hydrate (1:1) (2S)-3-(3,4-Dihydroxyphenyl)-2-hydrazino-2-methylpropanoic acid S-(−)-Carbidopa (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid hydrate (1:1) S-(-)-Carbidopa (-)-L-a-Hydrazino-3,4-dihydroxy-a-methylhydrocinnamic Acid (2S)-3-(3,4-dihydroxyphenyl)-2-hydrazinyl-2-methylpropanoic acid Hydrazino-a-methyldopa |
| Description | Carbidopa is an inhibitor of DOPA decarboxylase, which is used in parkinson disease.Target: DOPA decarboxylaseCarbidopa (CD), a competitive inhibitor of aromatic l-amino acid decarboxylase that does not cross the blood-brain barrier, is routinely administered with levodopa (LD) to patients with Parkinson disease (PD) to reduce the peripheral decarboxylation of LD to dopamine [1]. CD premedication improves 11C-5-HTP PET image quality and facilitates detection of NET lesions. Because of the similarity of metabolic pathways, this method could probably be applied to improve PET imaging using other tracers like 18F-DOPA and 11C-DOPA [2]. Carbidopa (100 microM) decreased growth of (but did not kill) SK-N-SH neuroblastoma and A204 rhabdomyosarcoma cells and did not affect proliferation of DU 145 prostate, MCF7 breast, or NCI-H460 large cell lung carcinoma lines. sublethal doses of carbidopa produced additive cytotoxic effects in carcinoid cells in combination with etoposide and cytotoxic synergy in SCLC cells when coincubated with topotecan [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 528.7±50.0 °C at 760 mmHg |
| Melting Point | 206 - 208ºC |
| Molecular Formula | C10H14N2O4 |
| Molecular Weight | 226.23 |
| Flash Point | 273.5±30.1 °C |
| PSA | 115.81000 |
| LogP | -0.19 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.641 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | MW5298000 |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |