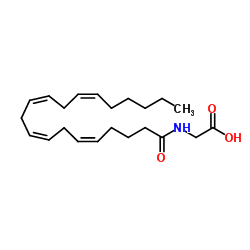
179113-91-8
| Name | N-Arachidonylglycine,N-(1-oxo-5Z,8Z,11Z,14Z-eicosatetraenyl)glycine |
|---|---|
| Synonyms |
N-[(5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoyl]glycine
NAGLY N-Arachidonoylglycine N-[(5Z,8Z,11Z,14Z)-5,8,11,14-Icosatetraenoyl]glycine arachidonoyl glycine N-ARACHIDONYL GLYCINE |
| Description | N-Arachidonylglycine (NA-Gly), a carboxylic analog of the endocannabinoid anandamide (AEA), is a GPR18 agonist (EC50 = 44.5 nM). Unlike AEA, N-Arachidonylglycine has no activity at either CB1 or CB2 receptors. N-Arachidonylglycine inhibits GLYT2 (IC50 = 5.1 μM). N-Arachidonylglycine also is an effective activator of endometrial cell migration[1][2]. |
|---|---|
| Related Catalog | |
| Target |
GlyT2:5.1 μM (IC50) |
| In Vitro | N-Arachidonylglycine (0.1 nM-100 µM; 5 min) drives MAPK activation in GPR18-transfected HEK293 cells[1]. N-Arachidonylglycine shows no activity at GLYT1 or GAT1 at concentrations up to 100 μm[2]. Western Blot Analysis[1] Cell Line: HEK293-GPR18 cells Concentration: 0.1 nM-100 µM Incubation Time: 5 min Result: Drove MAPK activation. |
| In Vivo | N-Arachidonylglycine (10 mg/kg; oral) increases blood concentrations of anandamide 9-fold[3]. N-Arachidonylglycine (1.2 mg/kg; oral; once) results in a significant 70% reduction of peritoneal cells[3]. Animal Model: Rats[3] Dosage: 10 mg/kg Administration: Oral Result: Inhibition of FAAH, causing a reduction in the hydrolytic cleavage of anandamid. Animal Model: Mouse (peritonitis model)[3] Dosage: 1.2 mg/kg Administration: Oral; once Result: Resulted in a significant 70% reduction of peritoneal cells. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 560.9±50.0 °C at 760 mmHg |
| Molecular Formula | C22H35NO3 |
| Molecular Weight | 361.518 |
| Flash Point | 293.0±30.1 °C |
| Exact Mass | 361.261688 |
| PSA | 66.40000 |
| LogP | 5.88 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.508 |
| Safety Phrases | S22-S24/25 |
|---|