74252-25-8
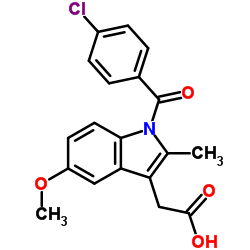
| Name | sodium,2-[1-(4-chlorobenzoyl)-5-methoxy-2-methylindol-3-yl]acetate,trihydrate |
|---|---|
| Synonyms |
Catlep
Indomethacinum Indomethacin sodium (USP) 1H-Indole-3-acetic acid,1-(4-chlorobenzoyl)-5-methoxy-2-methyl-,sodium salt,trihydrate Indomethacine {1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl-1H-indol-3-yl}acetic acid Durametacin UNII-0IMX38M2GG [1-(4-Chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl]acetic acid Dolcidium Indomethacin sodium trihydrate Chrono-indocid Sodium 1-(p-chlorobenzoyl)-5-methoxy-2-methylindole-3-acetate,trihydrate Indometacin sodium (JAN) indometacin Na salt,tri-H2O Indomethacin Indocin I.V. (TN) Chibro-amuno Bonidon |
| Description | Indomethacin sodium hydrate (Indometacin sodium hydrate) is a potent, blood-brain permeable and nonselective inhibitor of COX1 and COX2, with IC50s of 18 nM and 26 nM for human COX-1 and COX-2, respectively, in CHO cells[1]. Indomethacin sodium hydrate disrupts autophagic flux by disturbing the normal functioning of lysosomes[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Indomethacin is a potent and nonselective inhibitor of COX1 and COX2, with IC50s of 18 nM and 26 nM for human COX-1 and COX-2, respectively, in CHO cells. Indomethacin inhibits lipopolysaccharide (LPS)-induced PGE2 production (COX-2) in a human whole blood assay with a potency (IC50=0.68±0.17 μM), and suppresses coagulation-induced TXB2 production (COX-1) (IC50=0.19±0.02 μM). Indomethacin blocks COX-1 with an IC50 of 20±1 nM in U937 cell microsomes at a low arachidonic acid concentration (0.1 μM)[1]. |
| In Vivo | Indomethacin dose-dependently inhibits both the carrageenan-induced rat paw oedema (ED50, 2.0 mg/kg), hyperalgesia (ED50, 1.5 mg/kg), and is also effective at reversing LPS-induced pyrexia in rats (ED50, 1.1 mg/kg)[1]. Indomethacin (2.5 mg/kg, i.p) decreases the number of NeuN+ cells in the animals at 8 days after ET-1 injection. Indomethacin also reduces microglia/macrophage activation at 14 days. Indomethacin significantly increases the number of SVZ DCX+ cells/field at 14 days post stroke[3]. Indomethacin (22.9 mg/kg, p.o.) produces 8 to 10 linear mucosal lesions extended from the fundic to pyloric area of stomach wall[4]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 499.4±45.0 °C at 760 mmHg |
| Melting Point | 162ºC |
| Molecular Formula | C19H21ClNNaO7 |
| Molecular Weight | 357.788 |
| Flash Point | 255.8±28.7 °C |
| Exact Mass | 357.076782 |
| PSA | 99.05000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Storage condition | -20℃ |