93957-54-1
| Name | Fluvastatin |
|---|---|
| Synonyms |
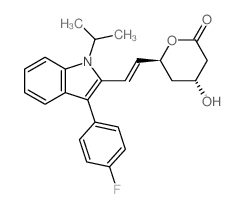
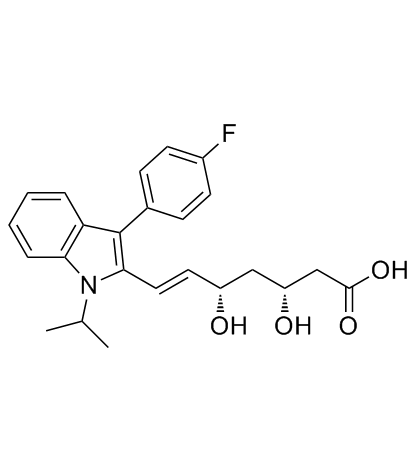
(3S,5R,6E)-7-[3-(4-Fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid
UNII:4L066368AS fluvastatin FLUVASTATIN NA Lipaxan MFCD00929076 (3R,5S,6E)-7-[3-(4-Fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid (3R,5S,6E)-7-[3-(4-Fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid Lescol (3R,5S,6E)-rel-7-[3-(4-Fluorophenyl)-1-(1-methylethyl)-1H-indol-2-yl]-3,5-dihydroxy-6-heptenoic acid (3S,5R,6E)-7-[3-(4-Fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid (3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid (±)-(3R*,5S*,6E)-7-[3-(p-fluorophenyl)-1-isopropylindol-2-yl]-3,5-dihydroxy-6-heptenoate FLUVASTATIN SODIUM Primexin |
| Description | Fluvastatin (Leschol) inhibits HMG-CoA reductase activity with IC50 of 8 nM.IC50 value: 8 nMTarget: HMG-CoA reductaseFluvastatin is a competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase (HMGCR), the enzyme that catalyzes the conversion of HMG-CoA to mevalonic acid, the rate-limiting step in cholesterol biosynthesis. Human hepatocellular carcinoma cell (HCC) studies indicate that Fluvastatin induces G2/M phase arrest. In the presence of Fluvastatin, HCC cells show a decrease of Bcl-2 and procaspase-9 expression, and an increase in Bax, cleaved caspase-3, and cytochrome c. Fluvastatin is antilipemic and is used to reduce plasma cholesterol levels and prevent cardiovascular disease. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 681.8±55.0 °C at 760 mmHg |
| Melting Point | 194-197ºC |
| Molecular Formula | C24H26FNO4 |
| Molecular Weight | 411.466 |
| Flash Point | 366.1±31.5 °C |
| Exact Mass | 411.184601 |
| PSA | 82.69000 |
| LogP | 3.62 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.587 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . R42/43:May cause sensitization by inhalation and skin contact . |
| Safety Phrases | S23-S26-S37 |
| RIDADR | UN 3272 3/PG 3 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 3 |
|
~% 
93957-54-1 |
| Literature: US2005/261354 A1, ; Page/Page column 53 ; US 20050261354 A1 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |