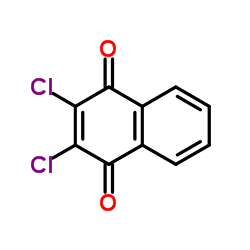
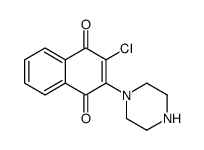
2797-51-5
| Name | quinoclamine |
|---|---|
| Synonyms |
Quinoclamin
Quinoclamine EINECS 220-529-2 2-AMINO-3-CHLORO-1,4-NAPHTHOQUINONE ACN 2-amino-3-chloro-naphthoquinone 2-Amino-3-chlor-1,4-naphthochinone 06K-Quinone 2-amino-3-chloronaphthalene-1,4-dione MFCD00001680 Mogeton 2-amino-3-chloro-1,4-dihydro-1,4-dioxonaphthalene ACNQ Mogeton granule O 6K-quinone 2-amino-3-chloro-1,4-naphthalenedione |
| Description | Quinoclamine, a naphthoquinone derivative, is a NF-κB inhibitor. Quinoclamine exhibits anti-cancer activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
NF-κB[2] |
| In Vitro | Quinoclamine causes differentiation of U-937 cells into macrophage-like cells[1]. Quinoclamine inhibits NF-κB activities in HepG2 cells, with an IC50 of 1.7 μM[2]. Quinoclamine (1-4 μM; 30 minutes ) suppresses endogenous NF-κB activity in HepG2 cells through the inhibition of IκB-α phosphorylation and p65 translocation[2]. Quinoclamine inhibits induced NF-κB activities in lung and breast cancer cell lines[2]. Quinoclamine affects the expression levels of genes involved in cell cycle or apoptosis[2]. Quinoclamine down-regulates the expressions of UDP glucuronosyltransferase genes involved in phase II drug metabolism[2]. Cell Viability Assay[2] Cell Line: HepG2 cells Concentration: 1 μM, 2 μM, 4 μM, 8 μM, 16 μM, 32 μM, 64 μM Incubation Time: 24 hours Result: Inhibited NF-κB activities in HepG2 cells. Western Blot Analysis[2] Cell Line: HepG2 cells Concentration: 0 μM, 1 μM, 2 μM, 4 μM Incubation Time: 30 minutes Result: Inhibited IκB-α phosphorylation and p65 translocation in HepG2 cells. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 310.2±42.0 °C at 760 mmHg |
| Melting Point | 198-200 °C |
| Molecular Formula | C10H6ClNO2 |
| Molecular Weight | 207.613 |
| Flash Point | 141.4±27.9 °C |
| Exact Mass | 207.008713 |
| PSA | 60.16000 |
| LogP | 1.31 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.660 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H319-H331-H400 |
| Precautionary Statements | P261-P273-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S45-S37/39-S26 |
| RIDADR | 2811.0 |
| RTECS | QL7350000 |
| HS Code | 2922399021 |
|
~77% 
2797-51-5 |
| Literature: Hodnett, Ernest M.; Wongwiechintana, Chinda; Dunn, William J.; Marrs, Pam Journal of Medicinal Chemistry, 1983 , vol. 26, # 4 p. 570 - 574 |
|
~95% 
2797-51-5 |
| Literature: Thapliyal, Prakash Chander Synthetic Communications, 1998 , vol. 28, # 7 p. 1123 - 1126 |
|
~% 
2797-51-5 |
| Literature: ENZON PHARMACEUTICALS, INC. Patent: US2011/305769 A1, 2011 ; |
|
~% 
2797-51-5 |
| Literature: Ullmann; Ettisch Chemische Berichte, 1921 , vol. 54, p. 271 |
|
~% 
2797-51-5 |
| Literature: Shishkina, R. P.; Berezhnaya, V. N.; Fokin, E. P. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science (English Translation), 1985 , vol. 34, # 10 p. 2160 - 2164 Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1985 , # 10 p. 2332 - 2337 |
|
~% 
2797-51-5 |
| Literature: Fries; Ochwat Chemische Berichte, 1923 , vol. 56, p. 1298 Full Text Show Details Ullmann; Ettisch Chemische Berichte, 1921 , vol. 54, p. 271 |
|
~% 
2797-51-5 |
| Literature: Fries; Ochwat Chemische Berichte, 1923 , vol. 56, p. 1298 Full Text Show Details Ullmann; Ettisch Chemische Berichte, 1921 , vol. 54, p. 271 |
| HS Code | 2922399021 |
|---|---|
| Summary | 2922399021 2-amino-3-chloronaphthalene-1,4-dione。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:30.0% |