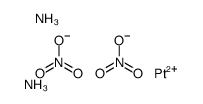
95734-82-0
| Name | nedaplatin |
|---|---|
| Synonyms |
(glycolato-O,O')diammine platinum(II)
2,2-diaMino-1,3-dioxa-2-platinacyclopentan-4-one Platinum, [2-(hydroxy-κO)acetato(2-)-κO]-, ammoniate (1:2) [(Hydroxy-κO)acetato(2-)-κO]platinum diammoniate Nedaplatin o(sup2))-diammine(hydroxyacetato(2-)-o(sup1(sp-4-3)-platinu cis-diammine(glycolato)platinum(ii) PlatinuM,diaMMine[(hydroxy-kO)acetato(2-)-kO]-,(SP-4-3) Nedaplait 254-s cis-diamine-glycolate-O,O'-platinum(II) |
| Description | Nedaplatin is a derivative of cisplatin and DNA damage agent.Target: OthersNedaplatin(NDP) is a derivative of cisplatin which produced less nausea & vomiting and nephrotoxicity. the effect of NDP on the 7-ethyl-1-hydroxy-CPT (the active form of CPT-11)-induced inhibitory effect on DNA topoisomerase I was examined. The topoisomerase I-inhibitory effect of 7-ethyl-1-hydroxy-CPT was enhanced 10-fold in the presence of NDP at microgram/milliliter concentrations [1]. NDP was developed as a second generation platinum complex. Because it has greater antitumour activity and lower nephrotoxicity than cisplatin (CDDP). At the high-dose of NDP in FN therapy, a reduction of tumour size and long-term tumour-free survival were frequently observed. The survival effect of the combinations of NDP with 5-FU was superior to those of the combination of CDDP with 5-FU. In conclusion, the sequence-dependent antitumour efficacy and toxicity of the combination of NDP or CDDP with 5-FU was demonstrated in this study, and FN therapy appeared to be the most efficient regimen as a clinical therapy [2]. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 265.6ºC at 760 mmHg |
|---|---|
| Molecular Formula | C2H8N2O3Pt |
| Molecular Weight | 303.181 |
| Flash Point | 128.7ºC |
| Exact Mass | 303.018280 |
| PSA | 42.01000 |
| LogP | 0.12010 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | Xn |
|---|
|
~28% 
95734-82-0 |
| Literature: Totani, Tetsushi; Aono, Katsutoshi; Komura, Michihiro; Adachi, Yasuko Chemistry Letters, 1986 , p. 429 - 432 |
|
~43% 
95734-82-0 |
| Literature: Totani, Tetsushi; Aono, Katsutoshi; Komura, Michihiro; Adachi, Yasuko Chemistry Letters, 1986 , p. 429 - 432 |
|
~% 
95734-82-0 |
| Literature: Chemistry Letters, , p. 429 - 432 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |