338-98-7
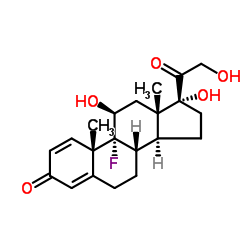
| Name | Isoflupredone Acetate |
|---|---|
| Synonyms |
(11b)-9-Fluoro-11,17-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl Acetate
21-Acetoxy-9-fluoro-11β,17-dihydroxypregna-1,4-diene-3,20-dione Pregna-1,4-diene-3,20-dione, 9-fluoro-11β,17,21-trihydroxy-, 21-acetate (8CI) (11β)-9-Fluoro-11,17-dihydroxy-3,20-dioxopregna-1,4-dien-21-yl acetate [2-[(8S,9R,10S,11S,13S,14S,17R)-9-fluoro-11,17-dihydroxy-10,13-dimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate 9-Fluoro-11β,17,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate 9-Fluoroprednisolone 21-acetate Pregna-1,4-diene-3,20-dione, 9-fluoro-11-β,17,21-trihydroxy-, 21-acetate 9α-Fluoro-11β,17α,21-trihydroxypregna-1,4-diene-3,20-dione 21-acetate Predef |
| Description | Isoflupredone acetate is a corticosteroids with anti-inflammatory activity. Isoflupredone acetate can be used for research ketosis, musculoskeletal disorders, hypersensitivity, infections, inflammatory diseases in cows, horse, pigs, et al.[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Isoflupredone acetate (10-4, 10-7, or 10-10 M; 48 or 96 h)mitigates the inflammatory and catabolic effects of IL-1β in synovial and osteochondral explants (from equine cadavers) to a greater extent in low concentrations (10-7 M and 10-10 M) than the high concentration (10-4 M)[1]. |
| In Vivo | Isoflupredone acetate (0.05 mg/kg; i.m.; on days 2 and 4 after the challenge) prevents dry-matter intake and average daily gain reductions, and leads to faster Mannheimia haemolytica-infected clinical improvement when co-treated with Oxytetracycline (HY-B0275)[2]. Animal Model: 96 weanling heifers (Bronchopneumonia was induced by intrabronchial infusion of Mannheimia haemolytica)[2] Dosage: 0.05 mg/kg Administration: i.m.; on days 2 and 4 after the challenge Result: Infection caused a reduction in dry-matter intake and average daily gain (ADG) in heifers.Prevented these reductions and resulted in faster clinical improvement when co-treated with Oxytetracycline (HY-B0275). |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.5±50.0 °C at 760 mmHg |
| Melting Point | 244-246 °C |
| Molecular Formula | C23H29FO6 |
| Molecular Weight | 420.471 |
| Flash Point | 302.5±30.1 °C |
| Exact Mass | 420.194824 |
| PSA | 100.90000 |
| LogP | 2.46 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.577 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~% 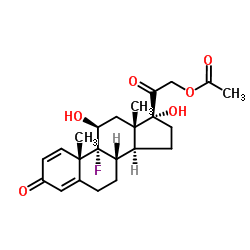
338-98-7 |
| Literature: Helvetica Chimica Acta, , vol. 39, p. 734,741 |
|
~0% 
338-98-7 |
| Literature: McLean; Khalil; Heiman; Lee Journal of Pharmaceutical Sciences, 1994 , vol. 83, # 4 p. 476 - 480 |
|
~% 
338-98-7 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4181 Journal of the American Chemical Society, , vol. 77, p. 3166 US2848464 , ; |
|
~% 
338-98-7 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4436 Journal of the American Chemical Society, , vol. 77, p. 4181 Journal of the American Chemical Society, , vol. 78, p. 4956,4959 |
|
~% 
338-98-7 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4181 |
|
~% 
338-98-7 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4181 |
|
~% 
338-98-7 |
| Literature: Journal of the American Chemical Society, , vol. 77, p. 4181 |
|
~% 
338-98-7 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 83, # 4 p. 476 - 480 |
|
~% 
338-98-7 |
| Literature: Helvetica Chimica Acta, , vol. 38, p. 1502,1506 |
| Precursor 7 | |
|---|---|
| DownStream 2 | |



![[2-[(8S,9R,10S,11S,13S,14S)-9-fluoro-11-hydroxy-10,13-dimethyl-3-oxo-7,8,11,12,14,15-hexahydro-6H-cyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate structure](https://image.chemsrc.com/caspic/272/1250-85-7.png)