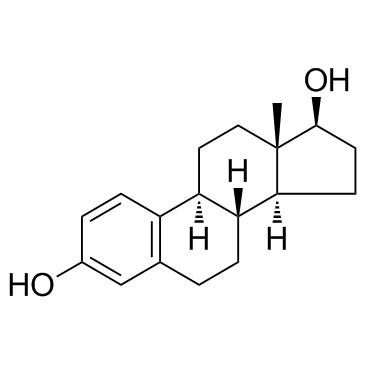
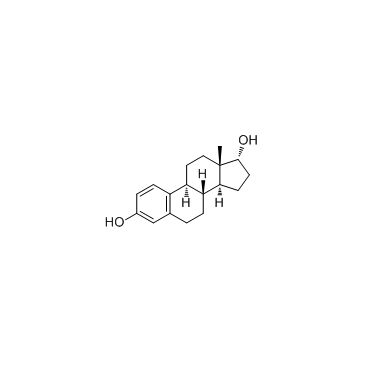
57-91-0
| Name | 17α-estradiol |
|---|---|
| Synonyms |
α-Estradiol
a-Estradiol Oestra-1,3,5(10)-triene-3,17α-diol alfatradiol MFCD00064144 (17a)-Estra-1,3,5(10)-triene-3,17-diol 3,17α-Dihydroxyestra-1,3,5(10)-triene trans-4-Hydroxycrotonic acid Epiestradiol Epiestrol Estra-1,3,5(10)-triene-3,17a-diol 3,17-α-Dihydroxyoestra-1,3,5(10)-triene (17α)-Estra-1,3,5(10)-triene-3,17-diol 3,17a-Dihydroxyestra-1,3,5(10)-triene Oestradiol-17a 3,17a-Dihydroxyoestra-1,3,5(10)-triene Oestra-1,3,5(10)-triene-3,17a-diol 17-Epiestradiol 17α-Oestradiol 3,17α-Dihydroxyoestra-1,3,5(10)-triene 17a-Estradiol 17α-Estradiol 17α estradiol Oestradiol-17α (8R,9S,13S,14S,17R)-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol Epiestradial EINECS 200-023-8 13b-Methyl-1,3,5(10)-gonatriene-3,17a-diol 17a-Oestradiol 17alpha-Estradiol 3,17-Dihydroxyestratriene Estradiol-17alpha |
| Description | Alpha-Estradiol is a weak estrogen and a 5α-reductase inhibitor which is used as a topical medication in the treatment of androgenic alopecia. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | Alpha-Estradiol (17 alpha-Estradiol) is a 5α-reductase inhibitor, and inhibits testosterone metabolism catalyzed by 5 alpha-reductase[1]. Alpha-Estradiol (17 Alpha-estradiol, 10 μM) attenuates LPS-induced inflammatory markers in both C57BL/6J male and female mouse embryonic fibroblast (MEF) cells, primary pre-adipocytes and differentiated 3T3-L1 adipocytes in an ERα-dependent manner, and such effects are through decreased NFκB-p65 and increased ERα protein expression[2]. |
| In Vivo | Alpha-Estradiol (17-alpha-estradiol, 0.01, 0.1, 1 μg) significantly reduces the percentage of central avascular/total retina area of the mouse pups. Alpha-Estradiol (1 μg) markedly decreasesmalondialdehyde (MDA) levels on postnatal days (PND) 9, 13, and 17 in retinas of hyperoxia-exposed pups. Alpha-Estradiol (1 μg) also decreases the number of NADPH-oxidase-positive cells, NADPH oxidase concentration and activity in retinas of the pups. In the 1.0-μg Alpha-Estradiol-treated pups, VEGF retinal concentrations are high on PND 9 but lower on PND 14 and 17. The best effect in retinas of 1.0-μg Alpha-Estradiol-treated pups is partly reversed by ICI182780 on PND 14 and 17[3]. |
| Cell Assay | Mouse embryonic fibroblast (MEF) cells are treated for the indicated time with Alpha-Estradiol (17 α-E2) or 17 β-E2 at 10 μM concentration. Inflammation is induced by LPS at a concentration of 10 ng/mL either alone or in combination with the respective estrogen[2]. |
| Animal Admin | Newborn mice are randomLy assigned to six groups according to the kind of treatment: room air with vehicle injection (control, group 1), hyperoxia with vehicle injection (control, group 2), hyperoxia with 0.01 μg Alpha-Estradiol injection (group 3), hyperoxia with 0.1 μg Alpha-Estradiol injection (group 4), hyperoxia with 1.0 μg Alpha-Estradiol injection (group 5), and hyperoxia with 1.0 μg Alpha-Estradiol and 10.0 μg ICI182780 injection (antagonist of estrogen receptor α and β) (group 6). The pups receive daily subcutaneous injections of either Alpha-Estradiol in vehicle [dissolved in ethanol and diluted in 0.05 mL/mouse of phosphate-buffered saline (PBS)] or vehicle alone from postnatal days (PND) 7-16. On PND 7, the pups in the hyperoxia and Alpha-Estradiol-treatment groups are exposed to hyperoxia (75 ± 2 % O2) for 5 days (PND 7-12) and then returned to normoxia (room air) for 5 days, along with the nursing mothers, whereas pups in the normoxia group are kept in normoxia from PND 7-17. The pups are humanely euthanized on PND 9, 13 (14), and 17[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 445.9±45.0 °C at 760 mmHg |
| Melting Point | 176-180ºC(lit.) |
| Molecular Formula | C18H24O2 |
| Molecular Weight | 272.382 |
| Flash Point | 209.6±23.3 °C |
| Exact Mass | 272.177643 |
| PSA | 40.46000 |
| LogP | 4.13 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Storage condition | 0-6°C |
| Water Solubility | ethanol: 50 mg/mL, clear, colorless |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS08 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H351 |
| Precautionary Statements | P281 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn: Harmful; |
| Risk Phrases | R40;R48 |
| Safety Phrases | 53-45-24/25-22 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | KG3750000 |
| Precursor 9 | |
|---|---|
| DownStream 1 | |


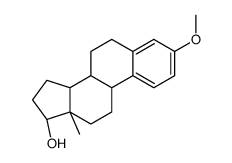
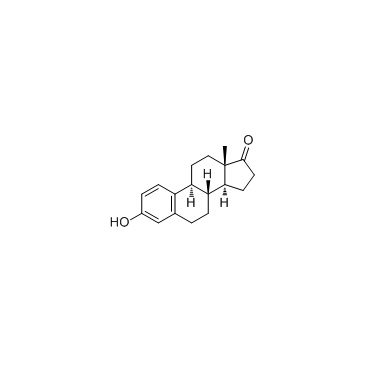

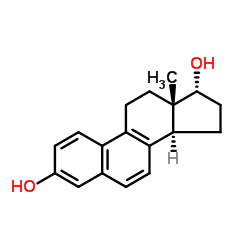
![N-[(E)-[(13S)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-ylidene]amino]-4-methylbenzenesulfonamide structure](https://image.chemsrc.com/caspic/314/55105-93-6.png)