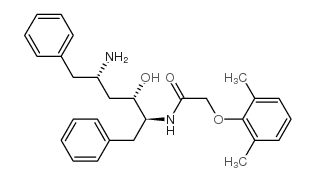
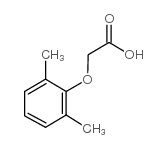
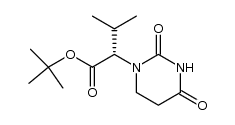
192725-39-6
| Name | Lopinavir Metabolite M-1 |
|---|---|
| Synonyms |
(2S,3S,5S)-2-(2,6-dimethylphenoxyacetyl)amino-3-hydroxy-5-(2S-(1-tetrahydropyrimid-2,4-dionyl)-3-methyl-butanoyl)amino-1,6-diphenylhexane
(aS)-N-[(1S,3S,4S)-4-[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2,4-dioxo-1(2H)-Pyrimidineacetami-de (aS)-N-[(1S,3S,4S)-4-[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]tetrahydro-a-(1-methylethyl)-2,4-dioxo-1(2H)-Pyrimidineacetami- d 4-Oxo-ABT-378 |
| Description | Lopinavir Metabolite M-1, an active metabolite of Lopinavir, inhibits HIV protease with a Ki of 0.7 pM. Lopinavir Metabolite M-1 has antiviral activities in vitro[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.7 pM (HIV Protease)[1] |
| In Vitro | Lopinavir Metabolite M-1 has antiviral activities in MT-4 cells, with an EC50 of 1.413 μM[1]. |
| References |
| Molecular Formula | C37H46N4O6 |
|---|---|
| Molecular Weight | 642.78400 |
| Exact Mass | 642.34200 |
| PSA | 137.07000 |
| LogP | 5.63440 |
| Index of Refraction | 1.582 |
|
~81% 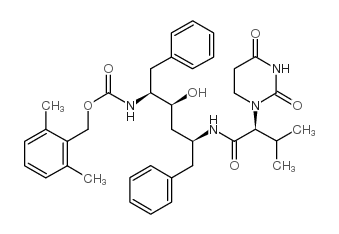
192725-39-6 |
| Literature: Sham, Hing L; Betebenner, David A; Herrin, Thomas; Kumar, Gondi; Saldivar, Ayda; Vasavanonda, Sudthida; Molla, Akhter; Kempf, Dale J; Plattner, Jacob J; Norbeck, Daniel W Bioorganic and Medicinal Chemistry Letters, 2001 , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
192725-39-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
192725-39-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
192725-39-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
192725-39-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
|
~% 
192725-39-6 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 11, # 11 p. 1351 - 1353 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |