15291-77-7
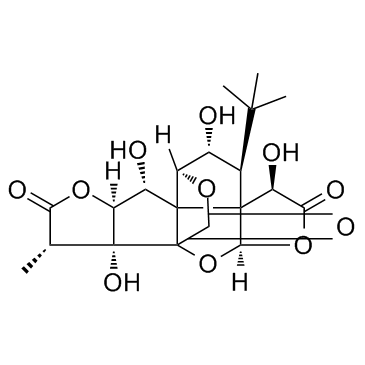
| Name | Ginkgolide B |
|---|---|
| Synonyms |
(1R,3R,6R,7S,8S,10R,11R,12R,13S,16S,17R)-6,12,17-Trihydroxy-16-methyl-8-(2-methyl-2-propanyl)-2,4,14,19-tetraoxahexacyclo[8.7.2.0.0.0.0]nonadecane-5,15,18-trione
ginkgolide b from ginkgo biloba leaves 7-DEOXYGINKGOLIDE C (1R,3R,6R,7S,8S,10R,11R,12R,13S,16S,17R)-8-tert-Butyl-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclo[8.7.2.0.0.0.0]nonadecane-5,15,18-trione 3-tert-butyl hexahydro-4,-7b-11 hydroxy-8-methyl MFCD05662347 (1R,3R,7S,8S,10R,11R,12R,13S,16S,17R)-8-tert-Butyl-6,12,17-trihydroxy-16-methyl-2,4,14,19-tetraoxahexacyclo[8.7.2.0.0.0.0]nonadecane-5,15,18-trione (1R,3R,6R,7S,8S,10R,11S,12R,16S,17R)-6,12,17-Trihydroxy-16-methyl-8-(2-methyl-2-propanyl)-2,4,14,19-tetraoxahexacyclo[8.7.2.0.0.0.0]nonadecane-5,15,18-trione ginkgolide B from ginkgo leaves GinkgolideB |
| Description | Ginkgolide B, an important active terpenoid from Ginkgo biloba leaves, is reported to increase cell viability and decrease cell apoptosis.IC50 value:Target:In vitro: Ginkgolide B (0.2 or 0.4 μg/ml) was added to the culture medium in vitro led to increases in cell viability and decreases in the number of hippocampal cells undergoing AAPH-induced apoptosis [1]. Ginkgolide B caused a dose-related protection against dysrhythmias; the antiarrhythmic effect of ginkgolide B was comparable to that of diltiazem and superior to the activity of metoprolol. Ginkgolide B can presumably prevent the re-entry mechanism involved in the development of ischemia-induced rhythm disturbances [2].In vivo: Oral administration of ginkgolide B (2 mg/kg/day, p.o.) caused a significant increase in cell viability and a highly significant decrease in the numbers of both spontaneously occurring and AAPH-induced apoptoses. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 762.4±60.0 °C at 760 mmHg |
| Melting Point | 280°C (dec.) |
| Molecular Formula | C20H24O10 |
| Molecular Weight | 424.399 |
| Flash Point | 274.3±26.4 °C |
| Exact Mass | 424.136932 |
| PSA | 148.82000 |
| LogP | 0.52 |
| Vapour Pressure | 0.0±5.8 mmHg at 25°C |
| Index of Refraction | 1.651 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
~95% 
15291-77-7 |
| Literature: Jaracz, Stanislav; Malik, Shahid; Nakanishi, Koji Phytochemistry, 2004 , vol. 65, # 21 SPEC. ISS. p. 2897 - 2902 |
|
~% 
15291-77-7 |
| Literature: Tetrahedron Letters, , vol. 33, # 46 p. 6955 - 6958 |
|
~73% 
15291-77-7 |
| Literature: Weinges, Klaus; Schick, Hartmut Liebigs Annalen der Chemie, 1991 , # 1 p. 81 - 83 |
|
~% 
15291-77-7 |
| Literature: Tetrahedron Letters, , vol. 33, # 46 p. 6955 - 6958 |
|
~% 
15291-77-7 |
| Literature: Tetrahedron Letters, , vol. 33, # 46 p. 6955 - 6958 |
|
~% 
15291-77-7 |
| Literature: Tetrahedron Letters, , vol. 33, # 46 p. 6955 - 6958 |
|
~% 
15291-77-7 |
| Literature: Liebigs Annalen der Chemie, , # 1 p. 81 - 83 |
|
~% 
15291-77-7 |
| Literature: Liebigs Annalen der Chemie, , # 1 p. 81 - 83 |
|
~% 
15291-77-7 |
| Literature: Bioorganic and Medicinal Chemistry Letters, , vol. 14, # 22 p. 5673 - 5675 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |