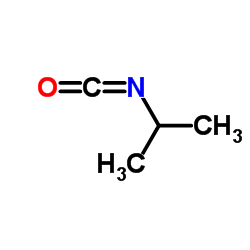
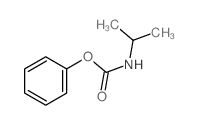
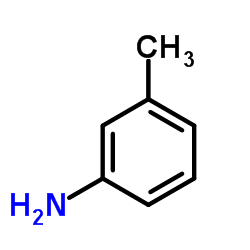
56211-40-6
| Name | torasemide |
|---|---|
| Synonyms |
N-[[(1-Methylethyl)amino]carbonyl]-4-[(3-methylphenyl)amino]-3-pyridinesulfonamide
Unat LUPLAC EINECS 200-659-6 Toradiur N-(Isopropylcarbamoyl)-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide Demadex Torem JDL-464 N-(Isopropylcarbamoyl)-4-[(3-methylphenyl)amino]-3-pyridinesulfonamide N-[(1-Methylethyl)carbamoyl]-4-[(3-methylphenyl)amino]pyridin-3-sulfonamid MFCD00866166 N-{[(1-methylethyl)amino]carbonyl}-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide GJ-1090 N-[(1-methylethyl)carbamoyl]-4-[(3-methylphenyl)amino]pyridine-3-sulfonamide AC-4464 1-Isopropyl-3-[(4-m-toluidino-3-pyridyl)sulfonyl]urea 3-Isopropylcarbamylsulfonamido-4-(3'-methylphenyl)aminopyridine Luprac Torsemide 4-[(3-methylphenyl)amino]-N-(propan-2-ylcarbamoyl)pyridine-3-sulfonamide Torasemide |
| Description | Torsemide is a pyridine-sulfonyl urea type loop diuretic.Target: OthersTorasemide is a pyridine-sulfonylurea type loop diuretic mainly used in the management of edema associated with congestive heart failure. It is also used at low doses for the management of hypertension. Torsemide significantly reduced total HF readmissions (relative risk [RR]: 0.41, 95% CI: 0.28-0.61, p < 0.0001) and HF readmissions (RR: 0.53, 95% CI: 0.33-0.84, p = 0.008) as well as CV readmissions (RR: 0.77, 95% CI: 0.60-0.98, p = 0.03) in patients with "at least 1 readmission." Torsemide caused a 14% reduction in all-cause mortality (RR: 0.86 [0.53-1.39], p = 0.54). Torsemide significantly reduces HF and CV-related hospital readmissions in systolic HF. Furthermore, torsemide is associated with a trend in reducing all-cause mortality [1]. Torsemide has several characteristics that make it suitable for treatment of advanced heart failure including longer half-life, increased potency of diuretic action, and anti-aldosterone effects. This case report details the administration of torsemide in 3 dogs with advanced heart failure and apparent furosemide resistance [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 163-164ºC |
| Molecular Formula | C16H20N4O3S |
| Molecular Weight | 348.420 |
| Exact Mass | 348.125610 |
| PSA | 108.57000 |
| LogP | 3.53 |
| Index of Refraction | 1.595 |
| Storage condition | -20°C Freezer |
| Water Solubility | Soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H319 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | UT7938000 |
| HS Code | 2935009090 |
|
~99% 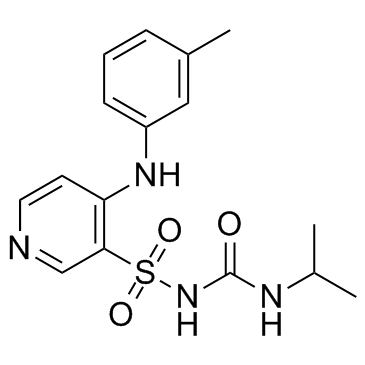
56211-40-6 |
| Literature: COSMA S.P.A. Patent: US2004/138469 A1, 2004 ; Location in patent: Page 4 ; |
|
~90% 
56211-40-6 |
| Literature: FINETECH LABORATORIES LTD. Patent: WO2003/97603 A1, 2003 ; Location in patent: Page/Page column 46-47 ; |
|
~% 
56211-40-6 |
| Literature: WO2004/9554 A1, ; Page 5-6 ; |
|
~% 
56211-40-6 |
| Literature: Arzneimittel-Forschung, , vol. 38, # 1 A p. 144 - 150 |
|
~88% 
56211-40-6 |
| Literature: Brantford Chemicals Inc. Patent: US2005/209460 A1, 2005 ; Location in patent: Page/Page column 3 ; |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |