CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XI0329000
-
CHEMICAL NAME :
-
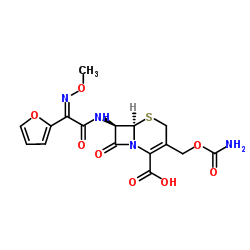
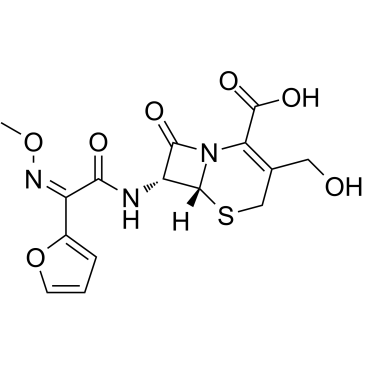
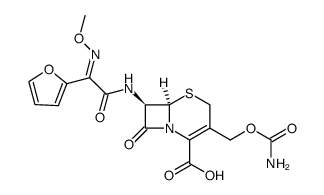
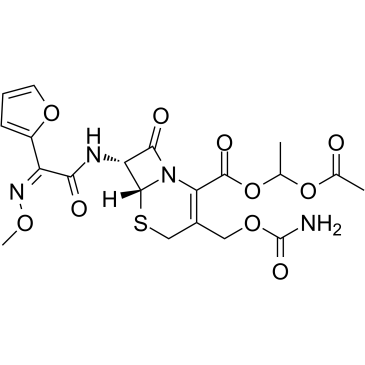
5-Thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 3-(((aminocarbonyl)oxy)methyl)-7-((2- furanyl(methyoxyimino)acetyl)amino)-8-oxo-, (6R-(6-alpha,7-beta(Z)))-
-
CAS REGISTRY NUMBER :
-
55268-75-2
-
LAST UPDATED :
-
199707
-
DATA ITEMS CITED :
-
19
-
MOLECULAR FORMULA :
-
C16-H16-N4-O8-S
-
MOLECULAR WEIGHT :
-
424.42
-
WISWESSER LINE NOTATION :
-
T46 ANV ES GUTJ G1OVZ HVQ CMVYUNO1&- BT5OJ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
64 mg/kg/16H-I
-
TOXIC EFFECTS :
-
Behavioral - toxic psychosis
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,965,1984
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>8 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - ataxia Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>10 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - ataxia
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
10400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DRUGAY Drugs. International Journal of Current Therapeutics and Applied Pharmacology Reviews. (ADIS Press International Inc., Suite B-30, Oxford Ct. Business Center, 582 Middletown Blvd., Langhorne, PA 19047) V.1- 1971- Volume(issue)/page/year: 17,233,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>2 gm/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - ptosis Behavioral - ataxia
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>1500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Lungs, Thorax, or Respiration - respiratory depression Gastrointestinal - hypermotility, diarrhea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
>300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),124,1979 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
30 gm/kg/5W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - changes in lung weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Blood - changes in erythrocyte (RBC) count
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),130,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
45 gm/kg/5W-I
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Blood - normocytic anemia Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),130,1979 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - other postnatal measures or effects
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),245,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
24 gm/kg
-
SEX/DURATION :
-
male 60 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),245,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
32 gm/kg
-
SEX/DURATION :
-
male 9 week(s) pre-mating female 2 week(s) pre-mating female 1-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),245,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
4400 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord)
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 27(Suppl 6),245,1979
|