Cefuroxime
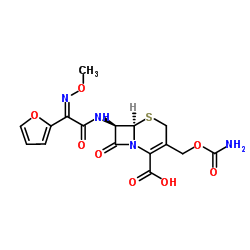
Cefuroxime structure
|
Common Name | Cefuroxime | ||
|---|---|---|---|---|
| CAS Number | 55268-75-2 | Molecular Weight | 424.385 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C16H16N4O8S | Melting Point | 171.5-173°C | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of CefuroximeCefuroxime is an orally active second-generation cephalosporin antibiotic with increased stability to β-lactamase. Cefuroxime has a broad spectrum activity against Gram-positive and Gram-negative bacteria[1]. |
| Name | cefuroxime |
|---|---|
| Synonym | More Synonyms |
| Description | Cefuroxime is an orally active second-generation cephalosporin antibiotic with increased stability to β-lactamase. Cefuroxime has a broad spectrum activity against Gram-positive and Gram-negative bacteria[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Cefuroxime is highly active against S. aureus (MIC=0.25 μg/ml), regardless of whether the strains produces a penicillinase. It is against Staphylococcus aureus methicillin susceptible; S. aureus, methicillin resistant, Streptococcus pyogenes, S. pneumoniae, S. viridans, S. faecalis, and Clostridium spp with MIC values of 0.25 μg/ml, 5.9 μg/ml, 0.125 μg/ml, 0.125 μg/ml, 0.125 μg/ml, >125.0 μg/ml, and 1.2 μg/ml, respectively[1]. Cefuroxime (10-100 μg/ml; 2-6 hours) rapidly bactericidal, its action is comparatively slow against the strains of S. aureus, but, even so, over 99% of the initial inoculum is killed by 6 h. The gram-negative organisms are killed rapidly, and in most cases over 99% of the very large inocula are killed within 2 h; the β-lactamase-producing strains are killed as quickly as non-enzyme-producing strains[1]. |
| In Vivo | Rabbits (weighing 2.0 to 2.5 kg) are challenged intravenously with S. aureus strain 630 (a penicillinase-producing strain), the median effective dose of Cefuroxime is 3 mg/kg as a result of the protection test[2]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Melting Point | 171.5-173°C |
| Molecular Formula | C16H16N4O8S |
| Molecular Weight | 424.385 |
| Exact Mass | 424.068878 |
| PSA | 199.06000 |
| LogP | 0.47 |
| Index of Refraction | 1.735 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R42/43 |
| Safety Phrases | 22-24-37-45 |
| RIDADR | NONH for all modes of transport |
| RTECS | XI0329000 |
|
~92% 
Cefuroxime CAS#:55268-75-2 |
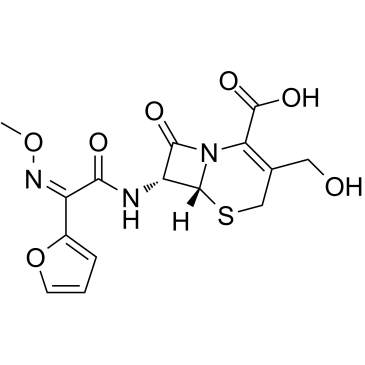
| Literature: Antibioticos S.p.A. Patent: US6642378 B1, 2003 ; Location in patent: Page/Page column 2-3 ; |
|
~71% 
Cefuroxime CAS#:55268-75-2 |
| Literature: Antibioticos S.p.A. Patent: US6642378 B1, 2003 ; Location in patent: Page/Page column 3-4 ; |
|
~% 
Cefuroxime CAS#:55268-75-2 |
| Literature: Journal of Pharmaceutical Sciences, , vol. 83, # 4 p. 553 - 558 |
|
Antibiotic exposure in a low-income country: screening urine samples for presence of antibiotics and antibiotic resistance in coagulase negative staphylococcal contaminants.
PLoS ONE 9(12) , e113055, (2014) Development of antimicrobial resistance has been assigned to excess and misuse of antimicrobial agents. Staphylococci are part of the normal flora but are also potential pathogens that have become ess... |
|
|
Beta- lactam antibiotics stimulate biofilm formation in non-typeable haemophilus influenzae by up-regulating carbohydrate metabolism.
PLoS ONE 9(7) , e99204, (2014) Non-typeable Haemophilus influenzae (NTHi) is a common acute otitis media pathogen, with an incidence that is increased by previous antibiotic treatment. NTHi is also an emerging causative agent of ot... |
|
|
Pharmacokinetics of cefuroxime in porcine cortical and cancellous bone determined by microdialysis.
Antimicrob. Agents Chemother. 58(6) , 3200-5, (2014) Traditionally, the pharmacokinetics of antimicrobials in bone have been investigated using bone biopsy specimens, but this approach suffers from considerable methodological limitations. Consequently, ... |
| EINECS 259-560-1 |
| cefuroxim |
| U1troxim |
| Ketocef |
| (6R,7R)-3-[(carbamoyloxy)methyl]-7-{[(2Z)-2-(furan-2-yl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| Cefuroxime |
| Biocidin |
| cefaloxime |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[[(aminocarbonyl)oxy]methyl]-7-[[(2Z)-2-(2-furanyl)-2-(methoxyimino)-1-oxoethyl]amino]-8-oxo-, (6R,7R)- |
| Cephuroxime |
| MFCD00864991 |
| CXM |
| Biofuroksym |
| (6R,7R)-3-[(Carbamoyloxy)methyl]-7-{[(2Z)-2-(2-furyl)-2-(methoxyimino)acetyl]amino}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| (6R,7R)-3-Carbamoyloxymethyl-7-[2-(2-furyl)-2-(methoxyimino)acetamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid |
| EkLxirrm |
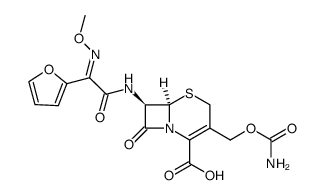
 CAS#:108-20-3
CAS#:108-20-3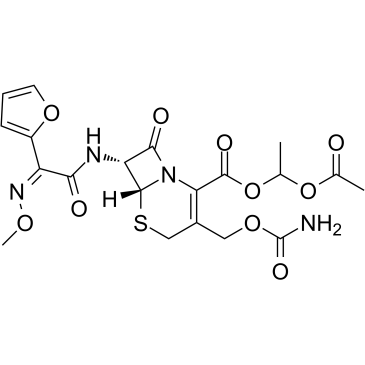 CAS#:64544-07-6
CAS#:64544-07-6